Introduction
With advancements in science and technology, chemicals are not only used for convenience in daily life, but also are used as necessary fuel and basic material in industries, such as those related to semi-conductors, electronics, cars, aerospace technology, information technology, biotechnology, and nanotechnology. However, in the case of improper handling, these chemicals may also be harmful substances to human health and the environment. Therefore, preventive measures have to be taken to protect workers who handle the substance. On the other hand, only less than 10% of 45000 domestically distributed chemicals have been studied for their potential risk, including chronic toxicity [1]. Consequently, chemical risk assessment has become more important as it functions as a scientific foundation for workplace management, such as classification of hazardous substances and the determination of threshold exposure levels in the workplace. Many characteristics of the workplace, including chemical properties, individual variances, working methods, exposure levels, and exposure durations, heavily influence the degree of chemical risk, so these factors should be taken into consideration by workplace managers [2]. Based on various legal requirements, as noted in the 39th regulation of, “Management of hazard substance”, the 81th enforcement regulations, “Classification and management of hazard substance” and “Determination of exposure limit”, the 40th regulation, “Examination of chemicals for risk and reproductive toxicity”, and the 41th regulation, “Equipment of material safety data sheet” in Occupational Safety and Health Act, precautions have been noted to protect the occupational health of workers from the hazard and unknown substances [3]. Therefore, to provide thorough protection against hazardous and unidentified substances, workplaces should take measures, including the anticipation and recognition of risk factors and determination of hazards and exposure levels of chemicals, to provide a scientific basis for decision making based on hazard information. The first step in the process would be to identify hazards, investigating the correlation between the chemical substance and human health. The second step would be to conduct a dose-response assessment, examining the correlation between the exposure level and human health. The third step would be exposure assessment, which evaluates the level of chemical substance exposure in the working environment. Lastly, the fourth step would be risk characterization to determine the overall level of risk. This study examined DEHP as it is commonly used by many manufacturers in industry and has a high toxicity, similar to plasticizers, such as polyvinyl chloride (PVC), plastic, and rubber, paint, and ink solvent. The toxicity of DEHP was evaluated, and the workplaces handling DEHP and the amount of DEHP handled were investigated. A dose-response assessment was performed to determine its toxicity, and an evaluation of the working environment re-examined the appropriate exposure level limit. The information obtained from this research could be utilized for the improvement of occupational health and the protection of workers handling DEHP.
Materials and Methods
Selection of Substance
DEHP was chosen as the chemical for investigation by the risk assessment board owing to its high levels of domestic circulation and the potential for harm to human health in case of mishandling.
Surveillance of Chemical Hazards
Hazard and risk assessment reports from international agencies, such as Organization for Economic Cooperation and Development Screening Information Data Set, International Agency for Research on Cancer (IARC), and International Program on Chemical Safety, and government databases, such as the National Institute of Occupational Safety and Health, US Environmental Protection Agency (EPA)’s Integrated Risk Information System, Japan Ministry of Health, Labor, and Welfare, and European Chemicals Agency were searched and examined to assess the risk DEHP poses to human health through both quantitative and qualitative analyses.
Toxicity Characterization
Based on the quantitative analyses from human and animal toxicity studies, parameters (e.g., no-observed-adverse-effect level [NOAEL], the lowest-observed-adverse-effect level [LOAEL], or alternative values) evaluating workers’ risk to chemical exposure in the workplace and DEHP carcinogenicity were investigated. Therefore, NOAEL and LOAEL were calculated for non-carcinogenic chemicals with the toxicity threshold, and the carcinogenic potential factor was estimated for carcinogenic chemicals without the toxicity threshold.
Assessment of the Exposure Level
The number of workplaces handling DEHP and the handling amount of DHEP were researched using the “Survey on the circulation amount of chemicals” by the Ministry of Environment and the Ministry of Knowledge and Economy’s international environmental regulations. DEHP exposure levels and working environments were assessed in five different workplaces including PVC and the chemical manufacturer. Each work site was visited to investigate work environments and conditions, and the use of local exhaust ventilation and respirators. The analysis of work environments was made according to the 2011-55 notification by the Ministry of Employment and Labor’s “Criteria for the measurement of working environment and evaluation of the site”, with emphasis on the handling process of DEHP.
Risk Characterization
Based on the toxicity value and the determined occupational exposure level, the probability of any harmful effect in the workers (risk) was calculated, and the validity of current management standards was evaluated using the Monte Carlo simulation, one of the tools used in hazard and risk assessment. Additionally, this method was further supplemented with a stochastic model to account for variance in DEHP exposure values caused by differences in working environment conditions. In other words, various variables affecting chemical exposure, such as DEHP handling and the working environment, the exposure concentration and distribution, the use of masks, and the exposure duration, were taken into account when determining the total amount of DEHP.
Results
Investigation of Di(2-ethylhexyl) Phthalate Characteristics and Circulating Amount
DEHP, also referred to as DOP, bis(2-ethylhexyl) phthalate, phthalic acid, and bis(2-ethylhexyl) ester, is a viscous and combustible liquid with a molecular weight of 390.54, specific gravity of 0.99, melting temperature of -46°C, boiling temperature of 385°C, vapor pressure of 3.4 × 10-3 torr (25°C), flash temperature of 215°C, explosive limit of 0.3%, and viscosity of 81.4 cp [4]. The number of workplaces, which either handle or manufacture DEHP, was 229, including rubber, plastic, and chemical substance manufacturing locations. The annual domestic circulation amount was found to be 831 tons, mostly consisting of vinyl chloride copolymer, PVC plasticizer, insecticides, dielectric of electric condenser, ink solvent, and oil for vacuum pumps.
Toxicity Evaluation
The 50% lethal dose (LD50), determined using acute oral toxicity testing, was found to be 23.6 g/kg for mice and 14.2 g/kg, 33.8 g/kg, and 50.0 g/kg for rats [5-7]. Moreover, the US National Toxicology Program (NTP) found the value to be higher than 20 g/kg in F344 rats after acute toxicity test [8]. For the sub-chronic toxicity testing, male and female rats were exposed to chemical inhalation at concentrations of 10, 50, 1,000 mg/m3 for four weeks, six hours each day, and five days per week. Female rats that received the highest concentration exhibited increased lung and liver weights, but recovered within eight weeks following exposure cessation. This observation may be explained by cellular organelle receptors for catalase enzymes, peroxidases, and oxidases, and the production of hydrogen peroxidase from molecular oxygen. The male rats, which were exposed to 1000 mg/m3 of the chemical showed hypertrophy of the lung cells [9].
In addition, chronic toxicity and carcinogenicity testing were performed in female F344 rats and male and female B6C3F1 mice, with ingested concentrations of 6000 and 12000 mg/kg, and 3000 and 6000 mg/kg for 103 weeks. The results showed that female rats and both male and female mice had a higher incidence rate of liver cancer. Male rats also exhibited an increase in the rate of liver cancer or neoplasm. Fifty-seven mice developed liver cancer, and 20 mice had lung metastases. Nine liver cancer cases found in male mice were reported to be within the normal range [8].
Based on these data, the NOAEL value for DEHP chronic toxicity and carcinogenicity was determined to be 28.9 mg/kg (rat) and the LOAEL was 146.6 mg/kg (rat). The IARC classified it as 2B, which indicates possible carcinogenicity in human, and based on sufficient evidence, DEHP was concluded to be carcinogenic in both the rat and mouse [10]. With regards to genotoxicity, the chromosomal aberration test results were all negative, and micronucleus test (in vivo assay) results were also negative as the observed DNA aberration did not have significant dependence on chemical concentration [11]. Using concentrations of 0.1, 0.5, 1.4, 4.8, 14, 46, 359, and 775 mg/kg/d, Sprague-Dawley rats were used to assess reproductive toxicity. Lesions in the testes were observed in a concentration dependent manner, and the NOAEL was determined to be 4.8 mg/kg/d. Additionally, Wistar rats were exposed to a range of concentrations, 0, 113, 340, and 1088 mg/kg/d, and two cases from the 1088 mg/kg condition were reported to have decreased testes weight. The rats in the 113 mg/kg concentration exhibited regional atrophy, and so, the LOAEL was determined to be 113 mg/kg/d [12].
To investigate the hazards of DEHP on human health, 54 workers in DEHP handling workplaces were grouped into 0.1, 0.2, and 0.7 mg/m3 concentration conditions based on their exposure amount in order to perform an epidemiologic survey. No correlation was found between exposure concentration and any health effects. Lung disease and lung function test results did not show any correlation to exposure to the test substance. In addition, the chromosome aberration test in 10 workers who handled DEHP for 10 to 30 years showed no increase in the chromosome aberrations when compared to the control group, and DEHP exposure level was also found to be low (0.09 to 0.16 mg/m3) [11].
Toxicity Characterization
The evaluations of DEHP exposure effects includes testing for acute toxicity, sub-chronic toxicity, chronic toxicity, and carcinogenicity toxicity, and this study conducted a dose-response assessment based on the chronic carcinogenicity test results of US NTP [8]. The test results of NTP revealed a NOAEL value of 28.9 mg/kg/d (rat), and the risk was determined using a linearized multistage procedure, which estimated the oral slope factor as 0.014 mg/kg/d. Based on this estimated oral slope factor and Korea Occupational Safety and Health Agency guide (W-6-2011) “Toxicity and risk assessment guideline of chemical substance”, the reference concentration for carcinogenicity risk, reference concentration in workplace (RfCwork) was determined. As shown in Table 1, “Calculation of RfCwork of DEHP”, the oral administration toxicity of 28.9 mg/kg/d was used to calculate RfCwork of carcinogenicity as 0.006 mg/m3 following steps 1 and 2. In contrast, when the oral slope factor determined by the US EPA (0.014 mg/kg/d) was used to determine the RfCwork of carcinogenicity, it was calculated to be 0.1 mg/m3, which was more than 10 times greater, and the risk of chronic toxicity and reproductive toxicity were evaluated as 0.264 and 0.330, respectively. This study used the RfCwork value of carcinogenicity with higher risk as reference, and the RfCwork value of 0.1 mg/m3, determined using the oral slope factor from the US EPA, was used in the dose-response assessment of DEHP. This minimizes the uncertainty between high and low concentrations.
Determination of Exposure Level
In order to determine the exposure level of DEHP, the working environments of 12 workers in DEHP manufacturing workplaces and 21 workers in DEHP handling workplaces were measured. As shown in Table 2, the DEHP manufacturing workplaces had an average exposure level of 0.158 ± 0.306 mg/m3, while DEHP handling workplaces had an average exposure level of 0.210 ± 0.257 mg/m3. The exposure levels in both places were below the exposure limit (5 mg/m3), but the workers in DEHP handling places seem to be exposed to higher levels of DEHP when compared to workers in DEHP manufacturing places when the data are represented in a box plot.
In other words, DEHP manufacturing and handling workplaces showed a mean exposure level of 0.191 ± 0.271 mg/m3, and the exposure levels ranged from no detection to 0.990 mg/m3. To check log-normal distribution of data, Shapiro and Wilk analysis was conducted as shown in Figure 1, and the straight line indicated significant log-normal distribution.
Risk Characterization
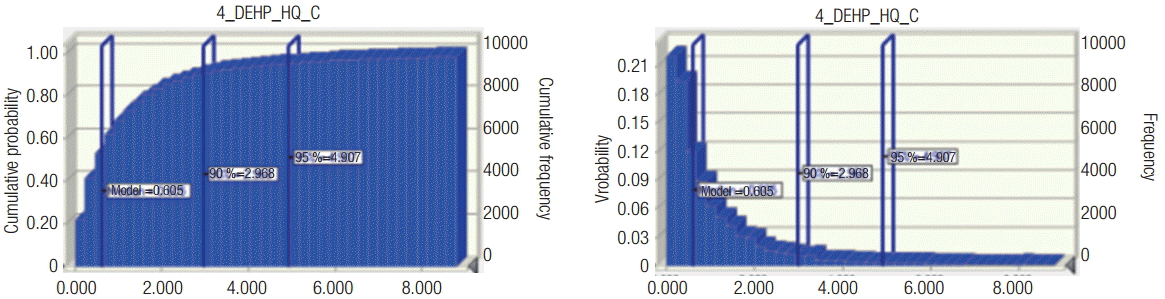
Risk was calculated by dividing the measured exposure level in the working environment by the reference concentration, RfCwork. In short, risk = measured value (mg/m3)/RfCwork (mg/m3). As shown in Table 1, the RfCwork value of 0.1 mg/m3 was used for the calculation of risk, and the carcinogenicity risk in DEHP handling workplaces was 1.3 on average, and the accumulative 95% value was 7.9, which is higher than the risk value of one. DEHP is a well-known endocrine disrupting substance, and its reproductive toxicity showed a similar pattern with chronic toxicity risk. The workers exposed to the high concentrations of DEHP were found to have a higher risk of occupational cancer, along with the potential risk of chronic toxicity and reproductive toxicity. In addition, the median probability distribution value of cancer in DEHP-exposed workplaces was 0.6, as shown in Figure 2, and the proportion of workers with potential exposure higher than the RfCwork value was estimated to be 35%. However, with regard to the chronic and reproductive toxicity of DEHP, the proportion of workers with potential exposure higher than the RfCwork was estimated to be 5% and 7%, respectively. Therefore, among domestic workers with DEHP exposure, the high concentration exposure group showed a high risk of carcinogenicity, and the potential risk of chronic toxicity and reproductive toxicity was determined to be lower than one.
Discussion
DEHP is a viscous liquid at room temperature with a subtle odor that poses as a fire hazard risk in the presence of ignition. According to toxicity testing, the acute oral toxicity lethal dose, LD50, was found to be 14.2 to 50.0 g/kg, which would be classified as an acute toxic substance level four (300 < acute toxicity estimate ≤ 2000 mg/kg) according to Globally Harmonized System of Classification and Labelling of Chemicals classification (low acute toxicity overall). However, chronic carcinogenicity testing revealed cases of lung and liver degeneration, atrophy of the testis, and liver cancer. The NOAEL value and LOAEL value were determined to be 28.9 and 146.6 g/kg, respectively, with potential reproductive toxicity and carcinogenicity. Based on sufficient evidence from rodent studies, DEHP was classified as a carcinogenic 2B substance by the IARC. During the absorption and metabolism of DEHP, mono(2-ethylhexyl) phthalate, mono(2-ethyl-5-oxohexyl) phthalate, and mono(2-ethyl -5-hydroxyhexyl) phthalate are generated and are known to be related to liver cancer [8]. Therefore, precautions should be taken when handling DEHP to prevent absorption via the respiratory system and skin. With regard to genotoxicity, the results from the chromosome aberration test and sister chromatid exchange test were negative, but some results from the micronucleus test (in vivo assay) and an in vitro assay showed positive chromosome aberration results. Therefore, despite inconclusive results, genotoxicity testing indicated the possibility of genetic effects based on in vitro exposure to DEHP [11]. The working environment measurements in DEHP handling workplaces were analyzed to calculate the minimum variance unbiased estimate for the lognormal distribution of 1.411 mg/m3, with land`s “exact” confidence limits of 0.584 to 32.60 mg/m3 and a 95th percentile point estimate of 4.720 mg/m3, which was below the exposure limit of 5 mg/m3. Although the working environment exposure value was lower than the exposure limit of 5%, the 95th percentile point’s confidence interval was 22.348 mg/m3, which exceeded the exposure limit. In addition, the exceedance fraction, which represents the portion of exposure above the exposure limit, was determined to be 4.79% with confidence interval of 1.77 to 11.30. Based on these results, the exposure limit of 5 mg/m3 could be considered an appropriate safety guideline value for DEHP. In addition, the dose-response assessments for chronic toxicity and reproductive toxicity were calculated to be 0.264 and 0.330, respectively, but the risk of carcinogenicity was 1.3, which exceeds the safety limit of one. Although the risk may vary depending on the toxicity parameters, DEHP’s risk of carcinogenicity existed at the concentration below the exposure limit. Therefore, the working environment should be appropriately managed to limit the exposure below 5 mg/m3 in order to protect the occupational health of workers handling DEHP. Operational measures, such as local exhaust ventilation installation and use of respiratory protection would also be recommended to minimize the exposure of chemicals via inhalation or skin.