Assessment of organophosphate pesticide residues in environmental media of Araromi farm settlement, Osun State, Nigeria
Article information
Abstract
The aim of the study was to assess the occurrence and distribution of organophosphate compounds residue in soil, surface water, sediment, and banana crops in Araromi farm settlement, Osun State, Nigeria. Organophosphate pesticide residues were determined using a gas chromatography equipped with Flame-Ionization Detection (GC-FID) in 16 soil samples from cocoa and banana farms, 6 water and sediment samples each, and 8 banana samples from 4 farms in the study site. Fourteen organophosphate compounds were detected (acephate, omethoate, dementon-s-methyl, dimethoate, tolcofos-methyl, pirimiphos-methyl, malathion, chlorpyrifos, methidathion, prothiofos, profenofos, ethion, azinphos-methyl and pyrazophos). Tolclofos-methyl, pirimiphos-methyl and prothiofos were detected in all the soil and sediment samples with concentration ranges of 1.9–12.9, 2.25–6.98 and 3.38–9.89 mg/kg respectively in soil and 8.13–9.83, 2.82–25.1 and 3.70–19.5 mg/kg respectively in sediment. Dimethoate, pirimiphos-methyl and prothiofos with concentration ranges, 0.06–0.28, 0.09–0.18 and 0.16–6.11 mg/L respectively were mostly detected in water samples while dimethoate, tolcofos-methyl, malathion, methidathion, prothiofos, ethion and azinphos-methyl compounds were detected in all the banana samples with concentration ranges, 3.40–12.0, 1.82–6.26, 5.73–9.48, 29.7–145, 8.24–20.1, 3.87–9.35 and 3.66–12.2 mg/kg respectively. The organophosphate mean residue concentrations were mostly significantly higher than the Maximum Residue Limits (MRL) at p<0.05. Across the three samples, only pirimiphos-methyl was significantly higher in water samples, omethoate in sediment; acephate, dementon-s-methyl and chlorpyrifos in banana were also not significantly higher at p<0.05. A strong positive significant correlation was observed between the organophosphate compounds in the banana and water samples (R=0.77, p=0.002) at p<0.05. The occurrence of organophosphate compounds in concentrations above MRLs may pose serious environmental and health risks.
Introduction
Pesticides play an important role in the control of wide range of human and livestock disease vectors, [1,2] and in the increase of crop yields. Several types of pesticides have been used in the last decades such as organochlorines, organophosphate, carbamates, pyrethrin and pyrethroids [3,4]. Organochlorine pesticides (OCPs) were commonly used as insecticides between the 1940s and 1970s [5,6] until they were banned at the Stockholm Convention on Persistent Organic Pollutants (POPs) in 2001 by the United Nations [7,8]. This action was due to their slow degradation and persistence in the environment [9,10]. After the ban of organochlorine, organophosphate pesticides (OPPs) became the most widely used pesticides accounting for an estimated 34% of world-wide insecticide sales [11,12]. This is because they degrade faster and are not persistent in the environment [13,14].
Pesticides are released into the environment during and after application. Over 90% of pesticides reach other destinations than their target sites [15]. They are spread in the air, soil, water and can be taken up by plants and animals. The movement of pesticides in the environment depends on the physical and chemical properties of the pesticides (such as volatility, water solubility and half-life) as well as environmental conditions [16]. The major route of pesticide spread is water, via surface runoff, disseminating it from application areas to other places in the environment.
The extensive use of pesticides by farmers has raised serious concerns about health risks resulting from residues in food and drinking water [17,18]. It is the nature of most pesticides to demonstrate a high degree of toxicity due to the mechanism by which they are intended to kill certain organisms [18]. On this basis, pesticide use has evoked great concerns not only of potential effects on sensitive ecosystems but also on human health [19,20]. There has been increasing evidence that human exposure to OPPs is linked to some health problems. Health impacts can range from acute, such as headaches, dizziness, and nausea to chronic like prolonged impairment of cognitive functions, including memory and attention [21].
Many researchers worldwide have monitored the levels of pesticide residues in soil and surface water [22–27] with varying levels of pesticide residues reported. Likewise, significant quantities of pesticide residues have been reported in cereals, vegetables and fruits [28,29]. In Nigeria, there have been evidences of the presence of OPP residues in soil [30,31], crops [30–33], and surface water [34–36]. The residue concentrations are above MRLs in most of these studies. Therefore, there is need for continuous monitoring of the environmental fate of these pesticides which can be used as basis for recommendation. The aim of this study was to monitor the concentration of OPPs in soil, surface water, sediment and banana crops in Araromi farm settlement, Osun State, Nigeria.
Materials and Methods
Study site
The study was conducted at Araromi farm settlement which is about 20 km from Ile-Ife town, with coordinates 7°35′00″N 4°37′00″E. It is one of the most important farm settlements of Atakumosa West Local Government, Osun State, Nigeria, producing cash and food crops such as cocoa, oil palm, cassava, banana and some leafy vegetables. The map of the study area and the sampling points are presented in Figure 1.
Sample size
The samplings of soil, water, sediment and banana were carried out two times during the dry season.
Collection and pre-treatment of soil samples
Four cocoa and four banana farms were selected purposively with distance to the Oloponyo and Opa streams. In each of these farms designated as banana farms A, B, C, D; and cocoa farms A, B, C, D; soil was collected from several sampling points to obtain a good representative sample. This implies that there were 4 samples each from cocoa and banana farms making a total of 8 samples and 16 for the two sampling periods. The soil samples were collected using a soil auger at depth 0–20 cm, stored in clean aluminium foil and wrapped with plastic bags for transportation to the laboratory. Afterwards, the soil samples were air dried to constant weight and sieved using 2 mm nylon mesh, the final sample for extraction was drawn using coning and quartering method.
Collection and pre-treatment of banana
Bananas were sampled across the four banana farms with the samples being collected from different sampling points. The samples were collected in clean polyethylene bag, labelled and transported to the laboratory. They were then peeled, sliced into small chips and oven dried at low heat to a constant weight and ground into flour. The samples were stored in labelled glass bottles in a refrigerator at 4 °C prior to extraction. There were 8 samples for the two sampling periods.
Collection and pre-treatment of water and sediments samples
Three water samples of about 2 L from three points namely Oloponyo Stream 1, Oloponyo Stream 2 and Opa Stream were collected with pre-cleaned glass bottles and were placed in an ice box to maintain the temperature at 4 °C during transportation. Sediments were also grabbed from the bottom of the stream at the same locations where water samples were collected in a clean aluminium foil wrapped with a plastic bag for transportation. At the laboratory, the water was kept in the refrigerator prior to extraction while the sediment samples were air dried to constant weight and sieved using 2 mm nylon mesh. There were 6 samples each for water and sediments for the two sampling periods.
Laboratory sample analysis procedure
Extraction of the samples
The extraction of the soil and sediment samples were done using the method described by Frimpong et al. [37], with slight modification adopted from Fosu-Mensah et al. [22]. 10 g of the representative soil samples were weighed and transferred into 250 mL separating flasks, 10 mL of acetonitrile was then added to each of the soil samples in the flasks and ultra-sonicated for 5 min. The flasks were closed tightly having added an additional 10 mL of acetonitrile. Thereafter, the samples were placed on a mechanical shaker to shake continuously for 30 min at 300 mot/min. The contents were then separated into layers when allowed to stand for 10 min. 10 mL of the supernatants and dried over 2 g anhydrous magnesium sulphate through filter paper into 50 mL round bottom flasks. The extract was reduced to about 2 mL using rotary film evaporator and made ready for silica clean up step.
The water samples were extracted by the method described by Fosu-Mensah et al. [22]. The water samples were filtered using WHATMAN filter paper, 1000 mL portions were drawn from the filtered water samples and transferred into 2 L capacity separating flasks with 30 mL of saturated sodium chloride solution (NaCl) added and mixed thoroughly by inverting the flask three to four times to produce a salt out effect in order to make the organophosphate more available for extraction in the non-polar organic layer. A 100 mL of dichloromethane as extraction solvent was thereafter added to each sample and manually shaken for 5 min, while releasing the pressure intermittently. The dichloromethane extracts (organic layers) were separated from the aqueous layers after it was settled. The extraction for each water sample was repeated thrice with 100 mL of dichloromethane and the organic layers put together and dried over anhydrous sodium sulphate through filter papers into 50 mL round bottom flasks. The extracts from the water samples were then concentrated to about 2 mL on rotary vacuum evaporators and prepared for silica clean up.
Extraction of pesticides from banana samples were carried out by EPA 3550C method as described by USEPA [38] using mixture of acetone and n-hexane (1:1 v/v) as the extracting solvent. To 20 g of banana sample was added 20 g of anhydrous Na2SO4 and mixed together into 250 mL conical flask. 50 mL of the solvent (mixture of acetone and n-hexane) were then mixed with the sample and sonicated in a high frequency ultrasonic bath for 15 minutes. The extract was then decanted into round bottom flask. The extraction process was repeated with additional 50 mL of the extracting solvent, sonicated and allowed to settle and decanted into the same round bottom flask. The combined extract was concentrated to 2 mL using a rotary evaporator and were re-dissolved in 5 mL n-hexane and later concentrated to 2 mL in a rotary evaporator.
Clean-up
The clean-up of the extracted samples was done using chromatographic columns packed with silica gel which have 2 g layer of anhydrous Na2SO4 on top and conditioned with the extracting solvent, except for banana samples that were conditioned with n-hexane. The concentrated extracts were then added into the column, and eluted with the extracting solvent. The eluates collected were dried using the rotary evaporator at 40 °C. The residues were re-dissolved in 1 mL ethyl acetate for soil, sediment and water samples, 1 mL n-hexane for the banana samples and transferred into gas chromatogram vials.
All extracts were kept frozen until quantification.
Quantification of the pesticide residues and their metabolites
The quantification of pesticide residues was performed using a gas chromatography equipped with Flame-Ionization Detection (GC-FID). The retention time, peak area and peak height of the sample were compared with those of the standards for the actual concentration of each analyte.
Data analysis
Statistical analysis of the data was performed using SPSS 26.0. The concentrations of OPP residues in the samples were presented in tables as means and standard deviation. The mean concentration of the OPP compounds across the various samples were compared with the maximum residue limits (MRL) using one sample t-test. All statistical tests are set at 5% confidence level (p<0.05).
Results
In this study, altogether fourteen (14) organophosphate compounds were detected across all the samples analyzed. The organophosphate compounds and their Chemical Abstracts Service (CAS) numbers are presented in Table 1.
Concentration of OPP in soil
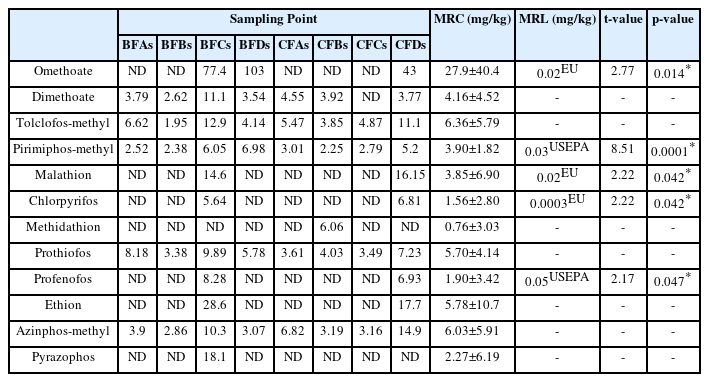
The concentrations of the pesticide residues detected in the soil are presented in Table 2. There were 12 OPP residues detected but the dominant compounds were tolclofos-methyl (1.95–12.9 mg/kg), pirimiphos-methyl (2.25–6.98 mg/kg) and prothiofos (3.38–9.89 mg/kg). The concentrations of prothiofos were highest in soils from banana farm A (BFAs) and banana farm B (BFBs) with values 8.18 and 3.38 mg/kg respectively, while omethoate was highest in soils from banana farm C (BFCs) and banana farm D (BFDs) with values 77.4 and 103 mg/kg respectively. In soils from cocoa farms, azinphos-methyl (6.82 mg/kg), methidathion (6.06 mg/kg), tolclofos-methyl (4.87 mg/kg) and ethion (17.7 mg/kg) were the highest in CFAs, CFBs, CFCs and CFDs respectively. The mean concentration of all the compounds compared across the samples were significantly higher than MRLs for agricultural soil at p<0.05 (Table 2).
Concentration of OPP in water
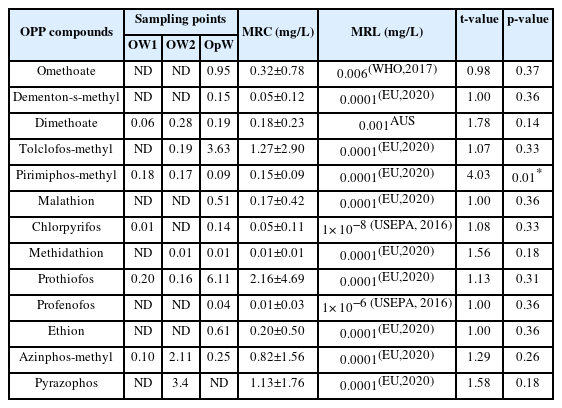
The concentrations of OPP residues detected in the water are shown in Table 3. The dominant compounds of the 13 OPP residues detected were dimethoate (0.06–0.28 mg/L), pirimiphos-methyl (0.09–0.18 mg/L) and prothiofos (0.16–6.11 mg/L). While pyrazophus was only detected in water from Oloponyo stream 2 (OW2) with mean value of 3.38±0.03 mg/L; dementon-s-methyl, profenofos, malathion and omethoate were detected only in water from Opa Stream (OpW) with values 0.15, 0.04, 0.51 and 0.95 mg/L respectively. The mean concentration of prothiofos was highest in water from Oloponyo stream 1 (OW1) and Opa Stream (OpW) with 0.20 mg/L and 6.11 mg/L respectively; while pyrazophos is highest in water samples from Oloponyo stream 2 (OW2) with 3.40 mg/L. The mean residue concentrations (MRC) of all the compounds were higher than the maximum residue limits (MRLs) in all the water samples but only the mean residue of pirimiphos-methyl (t=4.03, p=0.01) was significantly higher than the maximum residue limit at p<0.05.
Concentration of OPP in sediment
The concentrations of the pesticide residues detected in the sediment are presented in Table 4. There were 13 OPP residues detected but the dominant compounds were tolclofos-methyl (8.13–9.83 mg/kg), pirimiphos-methyl (2.82–25.1 mg/kg) and prothiofos (3.70–19.5 mg/kg). Acephate was only detected in sediment samples from Oloponyo stream 1 (OS1) with value of 8.30 mg/kg. Azinphos-methyl and tolclofos-methyl had the highest concentrations in sediments from OS1 and OS2 with 34.8 mg/kg and 8.13 mg/kg respectively, while the concentration of omethoate was the highest in sediments from OpS with 44.5 mg/kg. The mean residue concentrations (MRC) of all the compounds compared were higher than the maximum residue limits (MRLs) in all the sediment samples but only the mean residue of omethoate (t=1.58, p=0.18) was not significantly higher than the maximum residue limit at p<0.05.
Concentration of OPP in banana
The concentrations of the pesticide residues detected in the banana are presented in Table 5. There were 14 OPP residues detected with dimethoate (3.40–12.0 mg/kg), tolcofos-methyl (1.82–6.26 mg/kg), malathion (5.73–9.48 mg/kg), methidathion (29.7–145 mg/kg), prothiofos (8.24–20.1 mg/kg), ethion (3.87–9.35 mg/kg) and azinphos-methyl (3.66–12.2 mg/kg) compounds were dominantly detected in all samples. Acephate was only detected in banana from banana farm D (BFDb) with mean concentration of 4.39 mg/kg. The concentration of methidathion was the highest in bananas from Farm A (BFAb), Farm B (BFBb), Farm C (BFCb) and Farm D (BFDb) with 29.7, 32.0, 145 and 56.4 mg/kg respectively. The mean residue concentrations (MRC) of all the organophosphate compounds were all higher than the maximum residue limit (MRL) and were all statistically significantly higher than the MRL except for acephate (t=1.50, p=0.18), dementon-s-methyl (t=0.87, p=0.41) and chlorpyrifos (t=−1.69, p=0.14) at p<0.05.
Correlation of OPP residue in the environmental media
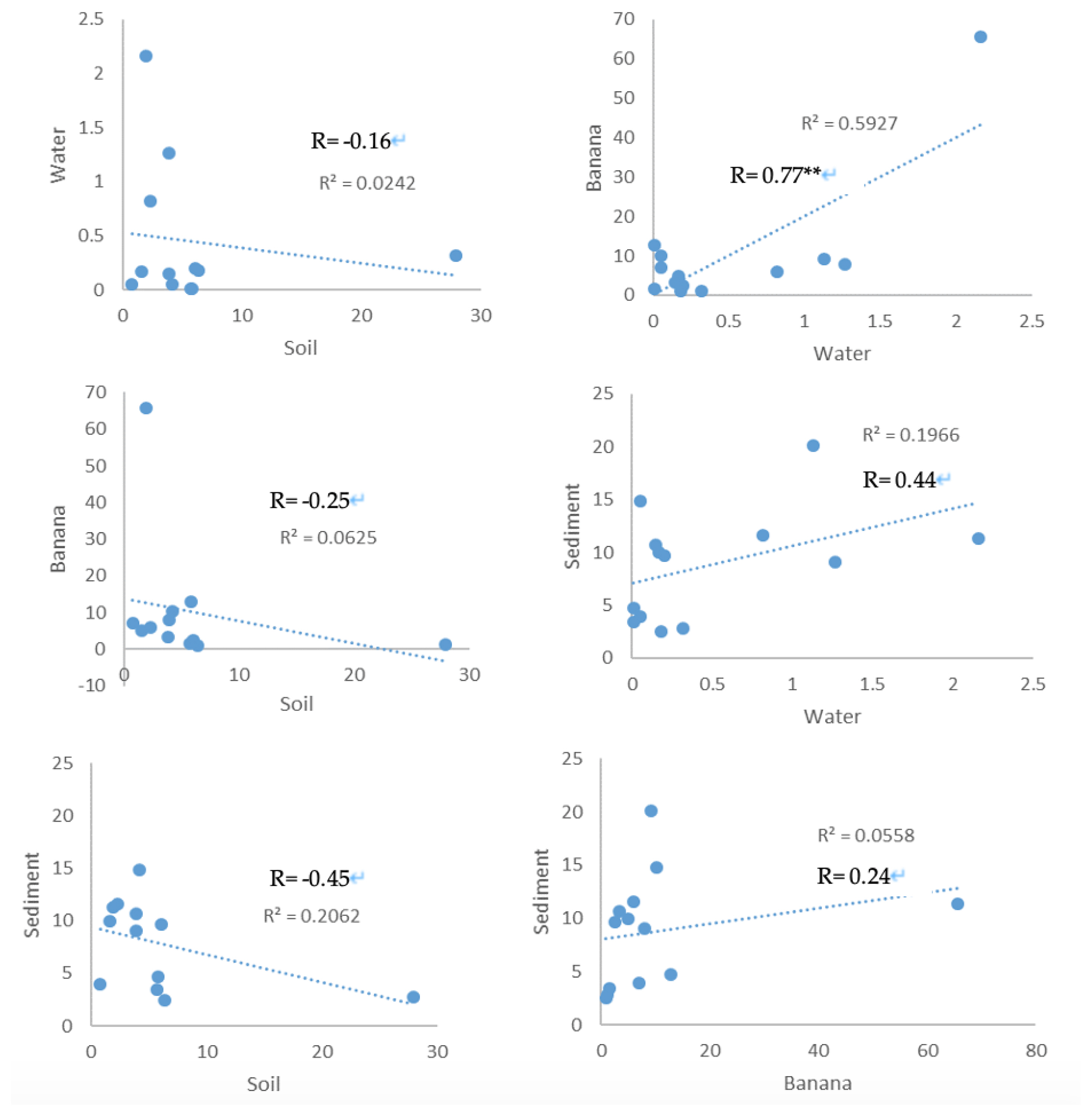
The organophosphate compounds in the soil samples were negatively weakly correlated with those in water (R=−0.16), banana (R=−0.25) and sediment (R=−0.45) samples with no significance at p<0.05 (Figure 1). A strong positive significant correlation was observed between the organophosphate compounds in the banana and water samples (R=0.77, p=0.002) at p<0.05 (Figure 2). Also, the organophosphate compounds in the sediment samples were positively weakly correlated with those in water (R=0.44) and banana (R=0.24) samples with no significance at p<0.05 (Figure 1).
Discussion
Most of the organophosphate compounds detected in this study have not been previously discussed in literatures especially in the studies carried out in Africa; therefore, this study may serve as a foundation for further studies on these compounds in the environment. The occurrence of many OPPs in the various samples may be because the farmers in this community are majorly into cash crops production like cocoa and oil palm which might have necessitated the use of different OPPs to improve their yield. The concentrations of the OPPs residues detected in the samples were generally higher than most MRL, this may be as a result of the fact that in most developing countries, farmers frequently apply pesticides above recommended concentration [25,39].
Metamidophos and dichlorvos were not detected in any of the samples. Dichlorvos may have been absent in the samples because of its higher water solubility which makes it degrade on time [25]. Even though metamidophos is absent; acephate which is considered its precursor was detected [25]. Omethoate and dimethoate were detected despite having higher water solubility which makes them degrade on time, this may be because they were applied frequently and recently at very high concentration [25,39]. The presence of chlorpyrifos is not surprising as it is considered an OPP with a broad-spectrum often applied on cocoa, which is the major cash crop in the farm settlement [22]. Methidathion and malathion were also detected across the samples; methidathion has been noted to be a non-systemic pesticide that is only persistent at lower temperature and pH below 7 and can easily run-off into surface water while the occurrence of malathion and its toxicity is dependent on their exposure to sunlight and ultraviolet light [40].
In the soil samples, the OPP residue concentrations were mostly higher than the MRLs allowed for agricultural soil. These occurrence and distribution in very high concentration may not be unconnected to various factors such as the pesticide’s mobility, persistence, and volatility; as well as the soil’s phosphorus and nitrogen levels, organic carbon content, and soil pH [25,39,41,42]. Bhandari et al. [25] have noted that most of the pesticides detected in soils have higher soil organic carbon-water partitioning coefficients (Koc), thereby increasing their chances of leaching into groundwater with attendant risk of pollution. The mean residue concentration of chlorpyrifos (1.56±2.80 mg/kg) detected is much higher than the range 0.01–0.06 mg/kg reported by Joko et al. [40] in soil samples from Wanasari subsdistrict, Brebes, Indonesia; 0.03±0.01 mg/kg reported by Fosu-Mensah et al. [22] in soil samples from cocoa producing areas in Ghana and 0.52–0.97 mg/kg reported by Mahmud et al. [31] in soil samples from Gashua, Yobe State, Nigeria. The high concentration of chlorpyrifos may be associated with its frequent usage by the cocoa farmers as well as its high soil absorption coefficient which increases its persistence in the environment [40,43]. The mean concentration of profenofos in soil (1.90±3.42 mg/kg) detected was higher than that of 0.03±0.01 recorded by Fosu-Mensah et al. [22] but relatively lower than 16.6±10.96 mg/kg reported by Harnpicharnchai et al. [44] from samples in Bueng Niam, Thailand. The mean concentration of ethion in soil (5.78±10.7 mg/kg) detected was also lower than 90±24.16 mg/kg reported by Harnpicharnchai et al. [44]. The mean concentration of 3.85±6.90 mg/kg for malathion observed in this study was higher than 0.137–0.363 mg/kg reported by Joko et al. [24], this comparison is also the same for methidathion with concentration of 0.76±3.03 mg/kg as against 0.014 mg/kg reported by Joko et al. [24], who noted that improper handling of pesticide formulations while mixing them, in addition to spillage, overuse and poor storage are responsible for their occurrence and persistence in the soil and that pesticide released into the atmosphere (in gaseous form) can return to the soil and water surface via wet deposition.
The occurrence of OPPs in the water samples may be as a result of transportation of volatilized pesticides atmospherically via wind into the water bodies, leaching and run-off, direct spillage, and improper disposal of pesticides container [22]. For the water, the mean concentration of 0.05±0.11 mg/L recorded for chlorpyrifos was higher than 0.04 μg/L reported by Fosu-Mensah et al. [22] in Ghana, and Malhat and Nasr [45] in water samples from the Nile River tributaries, in Egypt. The 0.15±0.09 mg/L mean concentration of pirimiphos-methyl was also higher than 0.03 μg/L reported by Fosu-Mensah et al. [22]. The mean residue concentration of 0.01±0.03 mg/L and 0.20±0.50 mg/L recorded for profenofos and ethion were less than 0.32±0.17 mg/L and 0.36±0.43 mg/L reported in the study by Harnpicharnchai et al. [44].
The OPP compounds which run-off into water bodies often sink into the sediments. The mean concentration of malathion (9.98±8.45 mg/kg) and Azinphos-methyl (11.6±18.0 mg/kg) in the sediment samples were higher than 0.05±0.01 mg/kg and 0.05±0.01 mg/kg respectively reported by Golshani et al. [46] in Tajan river. The mean concentration of Azinphos-methyl was higher than 0.57–2.29 mg/kg reported by Cembranel et al. [47] in urban lake located in Cascavel city, Brazil. The mean concentrations of pirimiphos-methyl (10.7±11.2 mg/kg) and profenofos (4.73±3.66 mg/kg) are higher than 0.104±0.052 mg/kg and 0.050±0.047 mg/kg reported by Akoto et al. [48] in sediments from Tono Reservoir.
Although cocoa is the main target of the application of the organophosphate pesticides in the farms, high concentration of the pesticides was detected in the banana samples. This is because bananas are cultivated close to cocoa farms and were contaminated either through absorption from soil or direct deposit from air. This agrees with Hendrichs et al. [15] who reported that over 90% pesticides reach other destinations than their target sites. They are distributed in untargeted crops, soil, water and air. The 9.11±9.10 and 0.93±1.72 mg/kg mean concentration of azinphos-methyl and dementon-s-methyl respectively reported in banana samples for this study was far higher than 0.31 and 0.05 μg/kg reported by Lemos et al. [49] from banana samples in Victoria, Brasil. Dimethoate residue with mean concentration of 7.96±9.68 mg/kg in banana samples was also higher than 0.004 mg/kg reported by Christia et al. [50] in Thessaloniki, Greece. These relatively high concentrations in the banana samples are of great concern because they are considered as one of the most important fruit crops in the world.
In this study, the occurrence of organophosphate pesticides in water, sediment and banana samples are positively associated with one other. This suggests that as these pesticides are applied to crops, they also find their way into the water bodies and eventually into the sediments. On the contrary, the mobility of the organophosphate compounds into the soil happens distinctly from all other samples. The organophosphate compounds residue concentration in soil, sediment and banana crop samples were generally higher than those in water samples. This is expected because the pesticides are applied directly to the crops and soil; therefore, the concentration of these compounds would have reduced due to their volatility before they run-off into surface water bodies while most are accumulated in the sediment.
Conclusions
The results of this study have provided an insight into the levels of OPPs residues contamination in the environment of Araromi Farm Settlements, Osun State, Nigeria. The presence of the fourteen OPPs detected suggests that the farmers use these pesticides because they are readily available and were registered for agricultural use in Nigeria, but used indiscriminately.
The occurrence of organophosphate compounds in the samples in concentrations above the MRLs may pose a serious risk and danger to soil and aquatic organisms and it is obviously something to worry about. From the soil, there is possibility of bioaccumulation and absorption into untargeted food crops via the roots which ultimately endanger humans who consume this produce.
The risk assessment of the organophosphate pesticides is therefore suggested to ascertain the level of exposure and hazards so as to ensure that prevention and control measures are put in place. There is also need for awareness, enlightenment and sensitization of the farmers and the entire community on proper and safe pesticides use.
Acknowledgement
My appreciation goes to the Pan African University Institute of Life and Earth Sciences (Including Health and Agriculture), University of Ibadan, Ibadan, Nigeria for funding this research as part of the requirement for the award of PhD in Environmental Management. I also express my gratitude to the chiefs of Araromi Farm Settlement who took me round the village and allowed me to collect samples in their farms.
Notes
Conflict of interest
The authors declare they have nothing to disclose.
CRediT author statement
YTA: Conceptualization, Methodology, Investigation, Resources, Formal analysis, Visualization, Writing-Original draft Preparation, Project administration; AYS: Conceptualization, Methodology, Writing-Review & Editing, Supervision; MBO: Conceptualization, Methodology, Writing-Review & Editing, Supervision.