A comparative study of efficacy of coconut oil, lemon water and dishwashing liquid as surrogates to xylene
Article information
Abstract
Histopathology is the field of science that helps in analyzing the architectural pattern of cells under the microscope. Hematoxylin and eosin-stained sections are used for routine histopathological examination. Xylene being a biohazardous hydrocarbon is used in many steps of tissue processing and laboratory personnel are exposed to this toxic substance. Maximum exposure to xylene occurs in the step of deparaffinization, for which alternate safer methods should be introduced. This study compares the efficacy of natural products like coconut oil, lemon water, less chemical substance like dish wash liquid with xylene as deparaffinizing agent. 50 paraffin embedded sections were used in each of the groups using xylene, coconut oil, diluted lemon water and dish wash liquid as deparaffinizing agents. 80% of slides using dishwashing liquid, 64% using lemon water and 42% of slides using coconut oil showed excellent cellular features. 96% of slides using xylene showed good quality staining, 54% of slides using dishwashing liquid and 40% slides using lemon water showed good quality staining. Only 4% of slides prepared using coconut oil showed good quality staining. Dishwashing liquid is the best surrogate and among the natural products, diluted lime water yields a better result and coconut oil, the least productive as deparaffinizing agent in this study.
Introduction
Histopathology is a study of analyzing the shapes, sizes and architectural patterns of cells and tissues using a microscope to appreciate the infected cells. This field of science is helpful in the specific clinical background for the knowledge and accurate way of diagnosis [1].
Fixation is the fundamental step in processing of histopathological slides. This step hardens the tissue and helps in further processing by maintaining the cell structure. Next step is Dehydration, where increasing concentration of alcohol is used to remove the water and fixative from the tissue. Clearing, the next step in which the dehydrant is replaced by an agent which is miscible with embedding medium paraffin. The most commonly used clearing agent is xylene as it has the same refractile index as that of proteins and maximum solvency displacing the alcohol and allowing maximum infiltration of paraffin. The next step is infiltration of the tissue with paraffin replacing the cleared tissue. This is followed by deparaffinization and staining. The commonly known deparaffinizing agent is xylene [2].
Xylene is colorless, sweet-smelling liquid and aromatic hydrocarbon that is frequently utilized as a solvent in industry and medical laboratories. It is found naturally in coal, wood tar, petroleum and it gets its name from the fact that it's present in crude oilwood.C6 H4 (CH3) 2 is the formula of the compound “Dimethyl benzene”, and it is made up of six carbon rings and two methyl groups [3]. The chemical xylene is used as a solvent in leather industries, printing, rubber, paint and in the product of cigarettes smokes and trace amount in fuel and gasoline. Xylene is used in paraffin section which is commonly used in H&E staining which stands the backbone of the histopathology laboratories and also in cytology Papanicolaou staining [4].
For almost 150 years, paraffin deparaffinization of the slides with xylene is the crucial step before the staining procedure because it allows the tissue sections to properly absorb the Hematoxylin and Eosin stain Because of its paraffin solvent effect, xylene is an unavoidable chemical in histology. It is the best deparaffinizing agent as it shows the accurate cellular structure of the tissues in H&E,Pap and various other special stains. The cell structure can be analyzed using morphometric analysis[5]. Xylene easily displaces the dehydrating agent and the molten wax causing minimal tissue damage. It has a high solvency capacity making it capable for excellent dewaxing and clearing agent [6].
Xylene is the most commonly used deparaffinizing and clearing agent but it has many known hazards to our health. Laboratory grade xylene is composed ofm-xylene (40–65%), p-xylene (20%), o-xylene (20%), and ethyl benzene (6–20%) with traces of toluene, thiophene, trimethyl benzene, phenol, hydrogen sulphide and pyridine [6].
There are various ill effects of xylene and the National Institute of Occupational Safety and Health (OSHA) has studied it in detail and has listed its recommendations. It has recommended acceptable exposure limits as 100 ppm for ten hours of continuous work exposure and a 40-hour exposure per week. A level of 200 ppm is acceptable only for a period of ten minutes. 7 more exposure can cause serious health hazards. A level of 200 ppm within a span of 3 to 5 minutes might cause irritation of the eyes, nose and throat. An accidental splash in the eye might cause redness, irritation and damage to the eye’s surface [7,8]. At high levels of exposure. Xylene can harm the lungs, liver, kidneys and the central nervous system. In lungs, in very severe exposure it causes pulmonary odema and shortness of breath. In the CNS, it affects the membrane proteins involved in the normal neuronal function [9]. Human blood cells like red blood cells, leucocytes or platelets have not been shown to be affected by xylene exposure. Anemia has no direct link to xylene but associated with benzene contamination of xylene [10].
The objective of this study is to use natural products like oconut oil (CO), diluted lemon water (DLW) and non-hazardous substance like dish wash liquid (DWL) as a replacement for deparaffinization by xylene in preparing H&E-stained slides and to compare the cellular morphology and staining characteristics among the slides. The novelty of the study is to compare the efficacy of the selected surrogates in their correct proportion as there are only few studies comparing their properties as dewaxing agents. This comparative study analyses the quality of staining, cost effectiveness and time period and disposal methods of the three.
Materials and Methods
This study was conducted using 4 groups by taking 50 random paraffin embedded blocks from the archives of Department of Pathology, Chettinad Medical College Hospital and Research Institute after obtaining Institutional Ethical Committee approval. It is an observational study. From each block 4 sections were cut and with a total of 200 slides with each group having similar set of 50 slides were used. The deparaffinizing agents used were xylene, coconut oil, 1.7% lemon water and 1.7% dish wash liquid.
Deparaffinization with xylene
Commercially available xylene was used for the study. Sections were dipped in steel container containing xylene I for 5 mins and xylene II for 5 mins, sections were rehydrated through graded alcohol and then washed in running tap water.
Deparaffinization with coconut oil
Sections were dipped in a steel container containing coconut oil at 90 °C for 2 mins and the washed with distilled water for 2 mins.
Deparaffinization with lemon water
1.7% lemon water is prepared by mixing 200 mL of concentrated lemon juice in 1500 mL of distilled water. Deparaffinization is done dipping the slide in prepared lemon water for 5 mins followed by distilled water for 2 mins. To neutralize the acidic nature of lemon water the slides are treated with lithium carbonate for 15 mins.
Deparaffinization with dish wash liquid
1.7% dish wash liquid is prepared by diluting 25 mL of commercially available dish wash in 1500 mL of distilled water. Deparaffinization done dipping the slide in prepared solution at 90°C for 5 mins, followed by wash in distilled water for 2 mins.
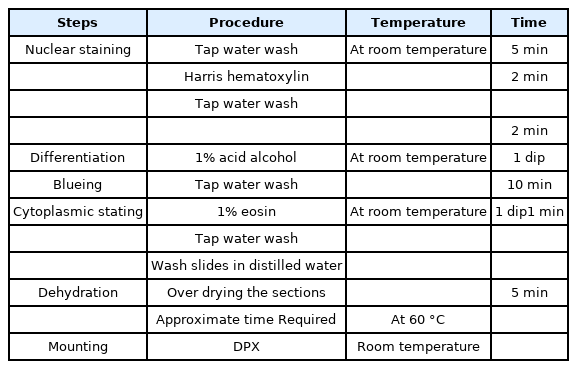
After deparaffinization the slides were stained by hematoxylin and eosin stain by the following procedure Table 1.
The temperature and time limit were set based on trial and error method. The slides were checked with quality of cellular architecture with score 0 and score 1 and quality of staining ranging from score 0 to score 2 with taking uniformity and crisp staining into account. The scores were obtained independently blinded by two pathologists.
1. Cellular architecture
SCORE 0: Indistinct nucleus - cytoplasm
SCORE 1: Distinct nucleus – cytoplasm
2. Quality of staining
SCORE - 0 = Poor
SCORE - 1 = Satisfactory
SCORE - 2 = Good
Results were tabulated and statistically analyzed using Chi-square test.
Results and Discussion
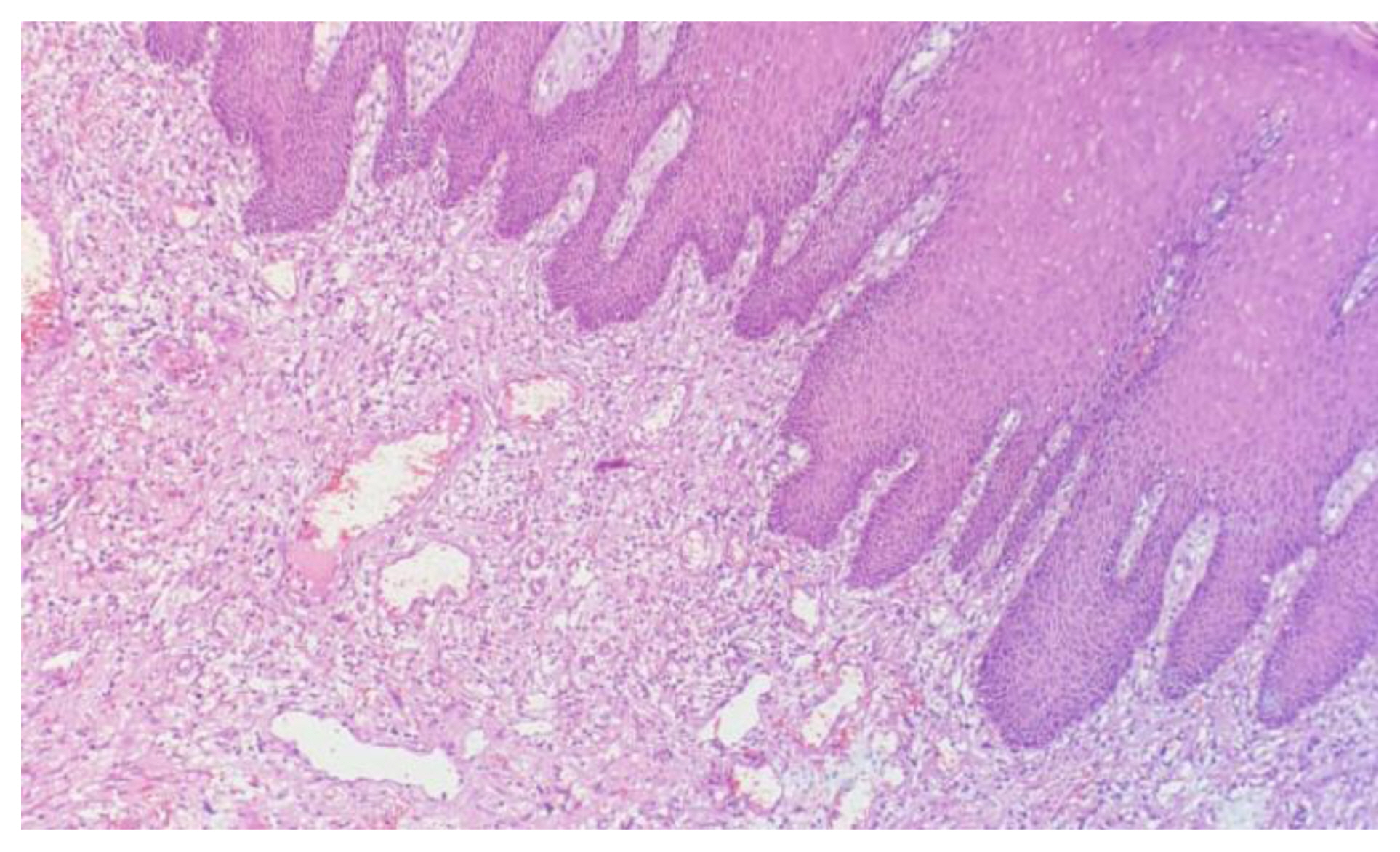
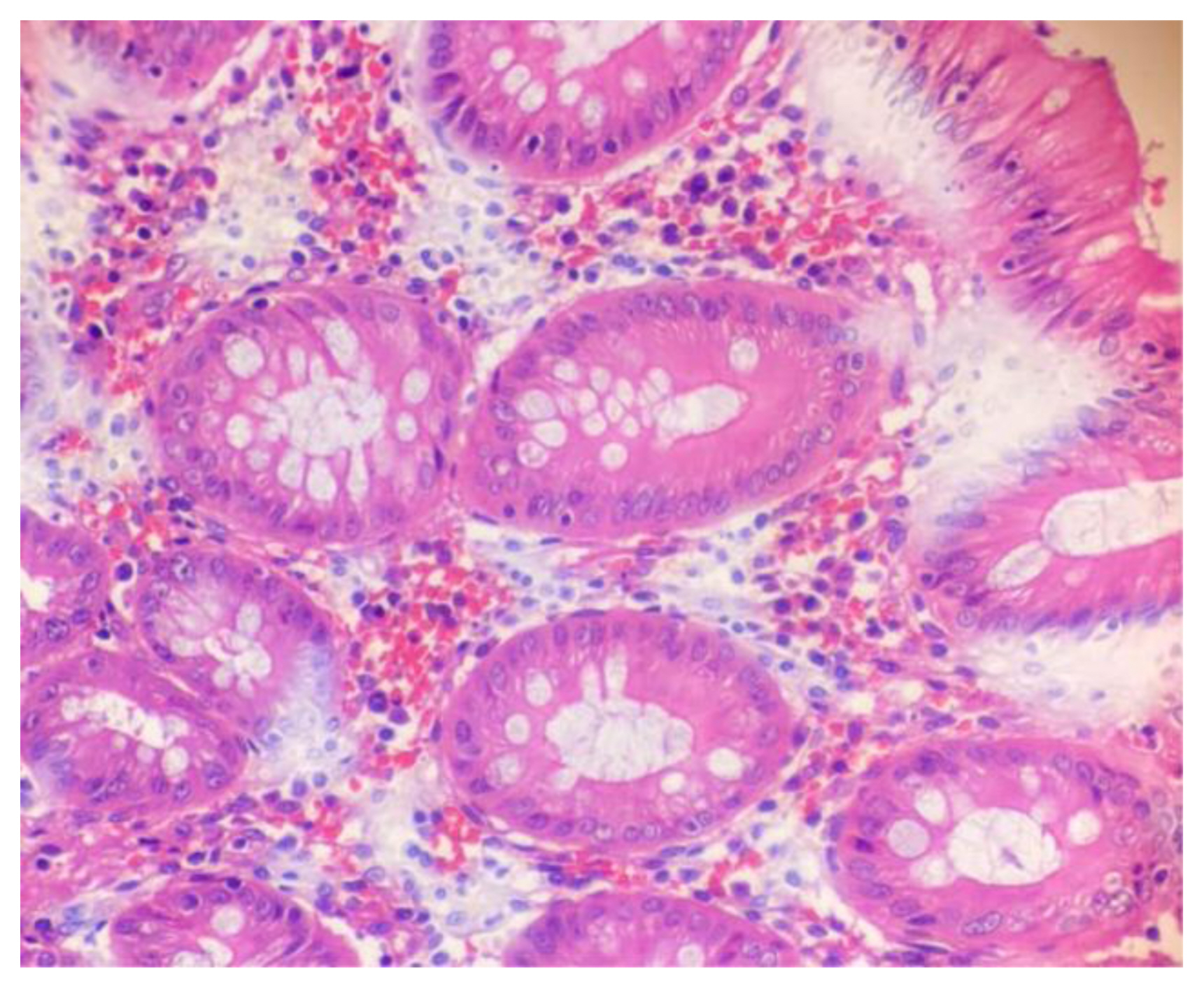
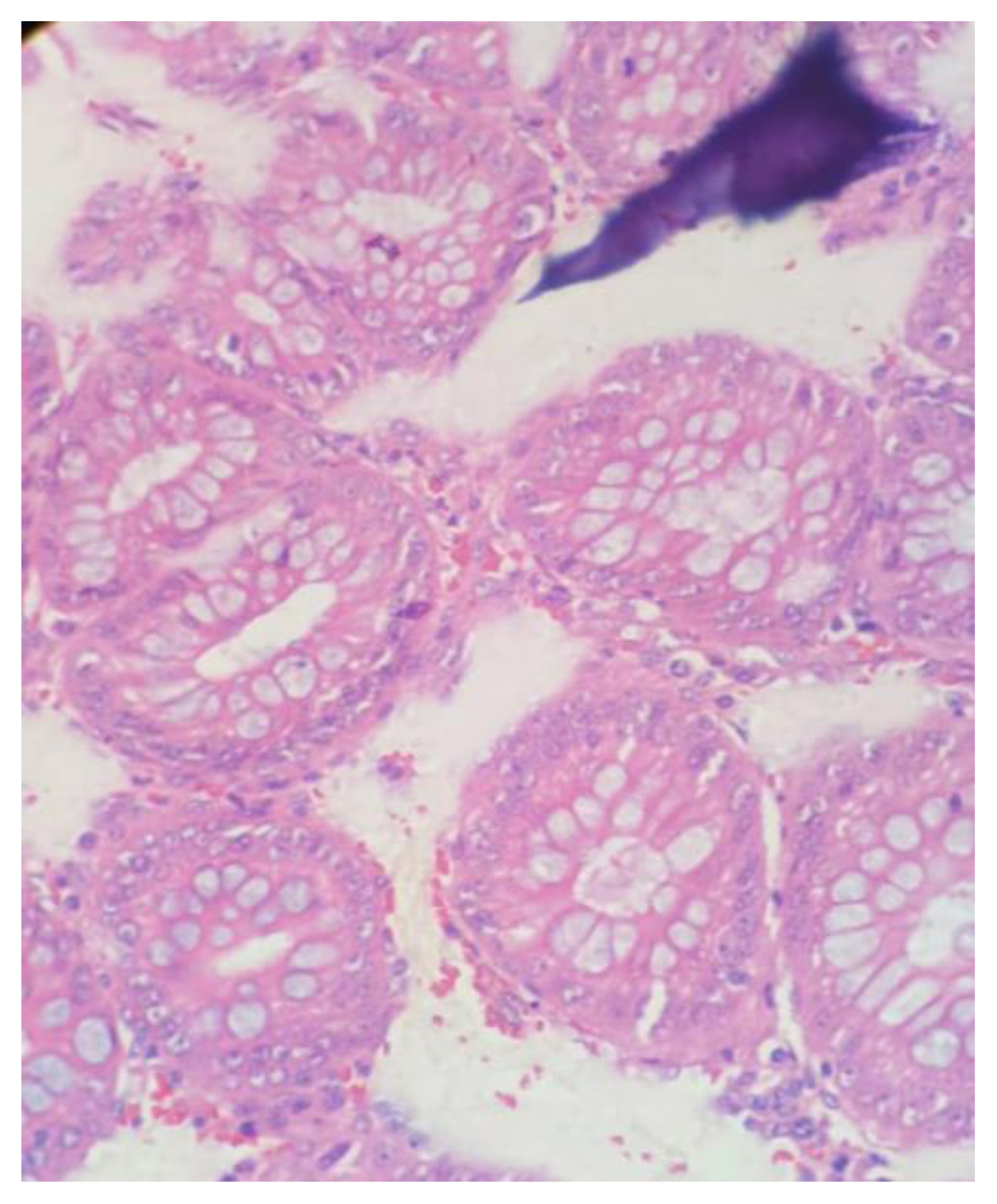
Figure 1 shows H&E stained slide using dishwash liquid as a clearing agent. It shows clear cellular architecture and proper staining with nuclear and cytoplasmic differentiation. Figure 2 shows slide stained using diluted lime water as clearing agent which also shows good cellular architecture with well-defined nuclear cytoplasmic differentiation. Comparing with images taken of slides using dilute lemon water and coconut oil as clearing agent as shown in Figure 3 and Figure 4, the staining characters and cellular architectures were better in slides stained using dishwash liquid.
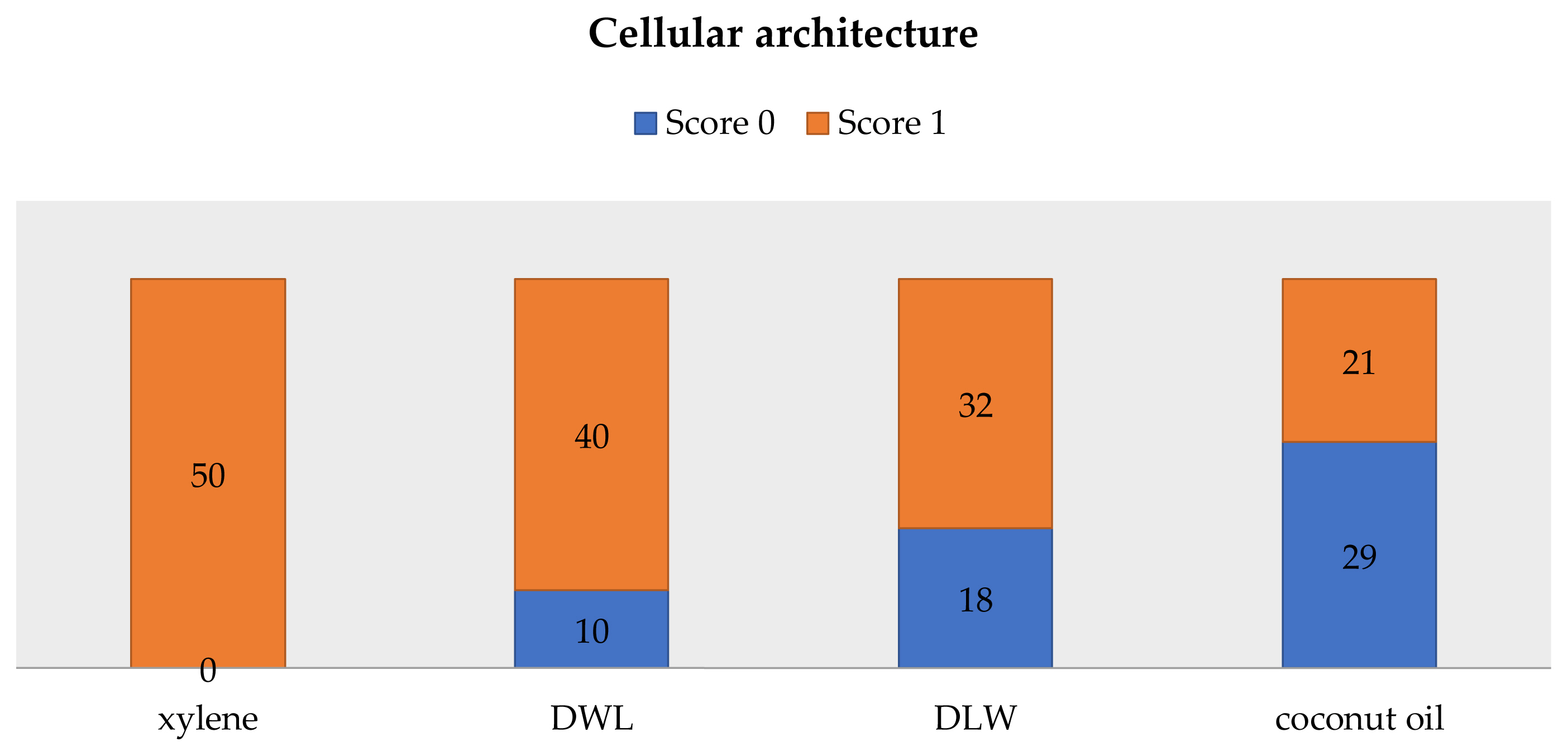
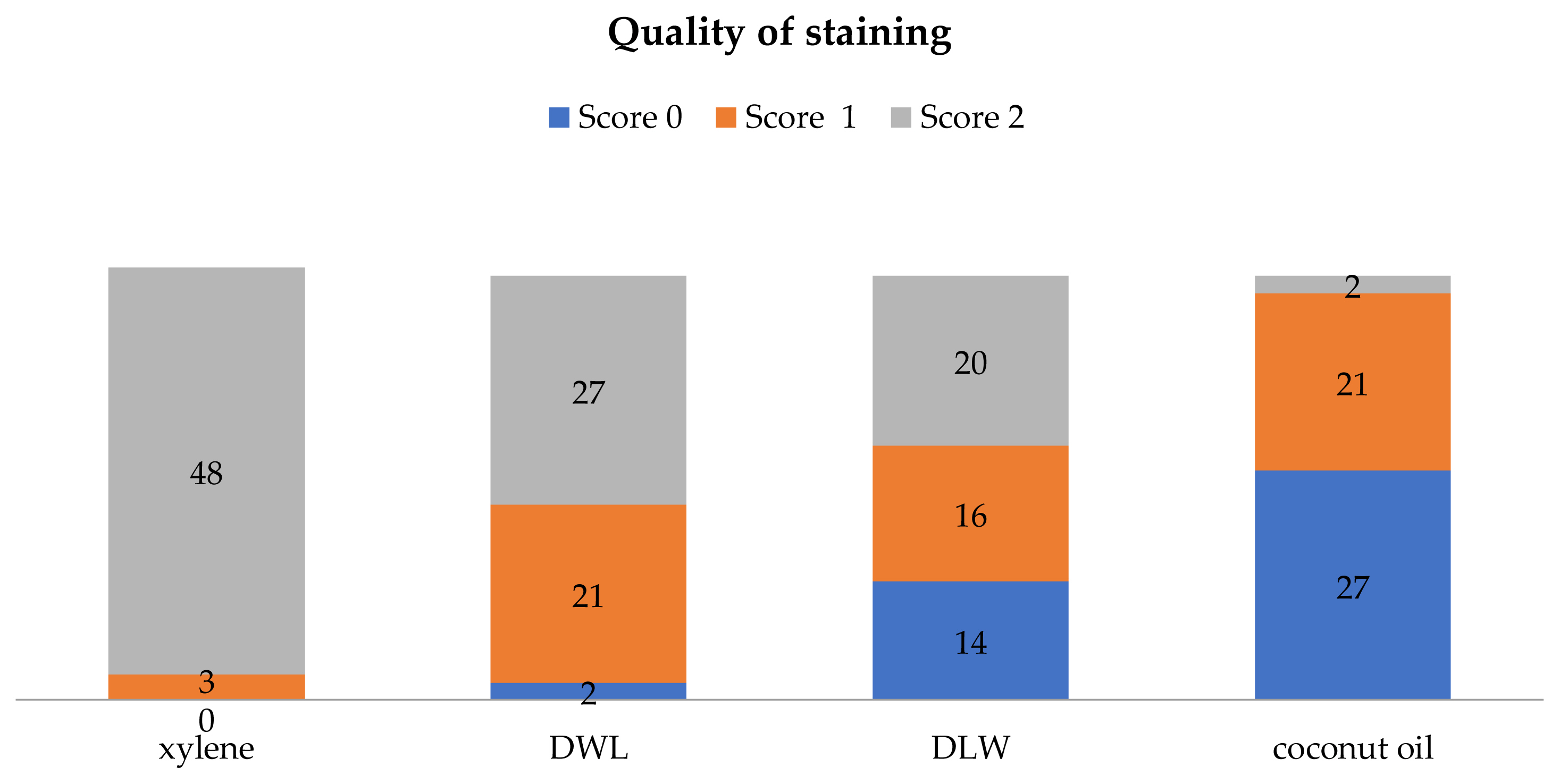
Out of the 50 samples, in 40 sections of dish wash liquid, 32 of lime water and 21 using coconut oil showed excellent cellular architecture. 80% of slides using dishwashing liquid, 64% using lemon water and 42% of slides using coconut oil showed excellent cellular features. 96% of slides using xylene showed good quality staining, 54% of slides using dishwashing liquid and 40% slides using lemon water showed good quality staining. Only 4% of slides prepared using coconut oil showed good quality staining.
Many authors have found that dish washing liquid to be a best first surrogative to xylene like in our study. It is principally made of alkylbenzene sulfonates, a forming mixture of nonionic surfactants, which causes less skin irritation. The mixture is used in hand washing of cutlery. The concept of using dish wash liquid as dewaxing agent is because of its surfactant property reducing the surface tension [11]. Some have highlighted the advantages of hot dishwashing soap (DWS) solution for deparaffinization of tissue sections for H&E staining and stated that even in special staining procedures like periodic acid-Schiff (PAS) staining and Van Gieson staining it can be applied. From trial and error method of increasing the concentration of dishwash liquid, 1.7% at 90 degree Celsius was found to be effective, as in other studies [12].
Sravya et al. [13] used nuclear, cytoplasmic, clarity, homogeneity, and crispness of staining to evaluate the effectiveness of DWL. One part was stained with traditional H&E (group A), whereas the other was stained with DWL procedure (group B). 94% of group A and 96% of group B sections had adequate nuclear staining, 92% of group A and 86% of group B sections had adequate cytoplasmic staining, 94% of group A and 96% of group B sections had clarity, 92% of group A and 80% of group B sections had uniform staining, and 96% of group A and 88% of group B sections had crisp staining, and 94% of group A sections stained adequately for diagnosis as compared with 90% in group B sections[13]. Ananthaneni et al. [14] have carried out a small investigation to see how effective dishwashing solution and diluted lemon water are at deparaffinizing sections for hematoxylin and eosin staining. A total score of 3–5 was considered appropriate for diagnosis, whereas a score of less than that was considered unsatisfactory. Both conventional and xylene-free H&E sections had better nuclear staining, crispness, and staining for diagnosis and concluded that DWL and DLW can be used instead of xylene [14]. Another study found that soapy sections had a statistically significant advantage in cytoplasmic (90%) and crisp staining (95%) and concluded that liquid DWL is a safe and effective alternative to xylene and alcohol in deparaffinization and routine H&E staining [15].
On the contrary, some studies found that lemon water to be effective in coloring the cytoplasmic and uniformity of tissues in the study than DWL, coconut oil [16]. The concept of using diluted lemon water as deparaffinizing agent was from its solvent property used to dissolve wax and prevents the wax from resticking onto the slides, thus helping in deparaffinizing the sections as a result, lemon water is a safe and effective substitute for xylene [17]. An Indian study stressed upon their finding in H&E staining, natural vinegar and diluted lemon water were found to be effective deparaffinizing agents [18]. Taking coconut oil into account, it showed poor quality staining. This is in accordance with other studies of Chandraker R et al. [20] and Sermadi et al. [19]. But certain studies of showed coconut oil to be better alternative [19]. Coconut oil is non-hazardous, less expensive and causes less shrinkage of the tissue. It is secure in heat, oxidises easily and has highest rancidity strength [20].
Previously Birgitte Bruun study investigated whether replacing xylene with olive oil or coconut oil affects the quality of histologic sections and found that only a minority of cases exhibited qualitative differences between xylene and oil-processed tissue [21]. Another study highlights that CO is non-hazardous, less expensive, and causes less tissue shrinking, the author demonstrates that it is a good substitute for xylene [22].
The knowledge of using natural, easily available substitutes like coconut oil, diluted lemon water and dishwash solution is essential small step for the future eco-friendly histopathological laboratories. All the three surrogates are very cost effective with a range of 900 to 50 Indian rupees. The time period ranges from 25 to 30 min of staining when compared to xylene which is 50–55 mins. All three are nontoxic, easy to prepare and dispose [23].
Comparison of cellular architecture is given in Figure 5 and comparison of quality of staining is given in Figure 6.
Conclusions
The objective of the study was to compare the efficacy of natural products of coconut oil and diluted lime water, dishwash liquid as a deparaffinizing agent during tissue processing and to find out the best biosafe alternative to xylene, thus reducing the use of harmful chemicals in histopathology laboratories. In this study, dishwash liquid yields the best result and other better alternative being diluted lime water.
Notes
CRediT author statement
AT: Conceptualization, Methodology, Investigation, Writing-Original draft preparation; SS: Data curation, Writing-Reviewing and Editing; GG: Visualization, Investigation, Software; AS: Writing-Original draft preparation.