Impact of polystyrene microplastic exposure on lipid profile and oxidative stress status of male and female Wistar rats
Article information
Abstract
Microplastics (MPs) are currently global environmental pollutants, and their associated health risks cannot be ignored. However, research on MP toxicity in mammals is limited. We investigated the effects of polystyrene microplastic (PS-MP) particles on the lipid profile and oxidative stress markers of Wistar rats. Two forms of PS-MP-pristine polystyrene and Styrofoam microplastics-particles of diameter <5 mm were used in this study. Each form was separately incorporated into rat feed in varying proportions of 1, 5 and 10%. A total of seventy rats (thirty-five male and thirty-five female rats) were used for this study and were separately distributed into seven groups of five rats each. The rats were then randomly assigned to a control group which received normal rat feed and water and six (6) test groups which were fed varying percentages (1, 5 and 10) of polystyrene microplastics diet for a period of 90 days. The rats were sacrificed under mild diethyl ether anesthesia 12 hr after cessation of treatment and blood was collected for lipid profile and oxidative stress analyses. Results obtained showed that oral exposure to microplastics caused decreased high density lipoprotein cholesterol (HDL-C) and increased low density lipoprotein cholesterol (LDL-C) in the rats. In contrast, there were no significant changes in oxidative stress parameters in the rats following microplastics exposure. Atherogenic indices in the PS-MP exposed rats differed according to gender. These results indicated that PS-MP dietary exposure may lead to dyslipidemia and male rats had higher cardiovascular risk.
Introduction
Microplastics (MPs) are generally considered to be plastic particles or fragments with a particle size of less than 5 mm [1] and are divided into two categories: primary MPs and secondary MPs [2]. Owing to their low production cost, good ductility, and durability, plastics are widely used worldwide. Between 1950 and 2015, approximately 6300 metric tonnes (MT) of plastic waste were generated, of which around 4900 MT was discarded in landfills or the natural environment [3]. Microplastic pollution exists widely, not only in the ocean, estuaries, rivers, lakes, and atmosphere, but also in the soil and indoor air [4–6]. Excessive MPs that enter the ocean and soil environments through multiple channels can affect the animals, plants, and microorganisms who live in these systems, and can even endanger human health through the delivery effect of the food chain/net [7]. Current research shows that MP inhalation [8], and digesting MP-polluted water and food (marine fish, shellfish, and vegetables) are the main pathways through which MPs enter the human body [9,10]. Microplastic pollution is listed as an important scientific issue in the fields of environment and ecology, and the health risks caused by it have attracted increasing attention [11]. At present, most studies on the biological toxicity of MPs have mainly focused on marine and aquatic animals, such as various fish species and invertebrates [12]. However, there are a few studies on the health risks of MPs to organisms at the highest trophic level, such as marine mammals and terrestrial mammals, and even humans. Mice are a commonly used mammalian model in medical research. Deng et al. [13] conducted a study and found an accumulation of 5 μm and 20 μm fluorescent and pristine polystyrene microplastic (PS-MP) particles in mouse liver, kidney, and gut. In addition, biochemical and metabolomics analyses indicated that MP exposure disturbs energy and lipid metabolism and causes oxidative stress and alterations in neurotoxicity biomarkers. Two other studies also revealed that micron level MPs could accumulate in the gut of mice and induce intestinal barrier dysfunction, gut microbiota dysbiosis, and metabolism disorder in mice [14,15]. Recent studies also showed that MPs caused pyroptosis and apoptosis of ovarian granulosa cells [16], and inflammatory effects [17], and lead to reproductive toxicity or behavioral disorders [18,19]. As orally administered MPs can be detected in the intestine, liver, and kidney, they likely enter the body’s organs through blood circulation, and their effects on the biochemical profiles of mammals have received attention lately. However, what is not yet clear is if the impact of microplastics exposure is gender dependent. Given the lack of information concerning the potential gender dependent effects of PS-MPs in mammals, this study aimed to comparatively examine the impact of polystyrene microplastic exposure on lipid profile and oxidative stress markers of male and female Wistar rats.
Materials and Methods
Chemicals and reagents
All chemicals and reagents used are of analytical grade. Commercial reagent kits for the assay of total cholesterol (TC), total protein (TP), triglyceride (TAG), high density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were as supplied by Randox Diagnostics, Crumlin, UK.
Experimental animals
Seventy male and female Wistar rats with weight between 120 and 150 g were obtained from the Experimental Animal House of the Department of Biochemistry at the University of Port-Harcourt, Nigeria. The animals were kept in well ventilated cages at room temperature (28–30 °C), and under controlled light cycles (12 h light/12 h dark). They were allowed to acclimatize for a period of 7 days before the commencement of treatments. The rats had free access to standard rat chow and clean water ad libitum. Handling of animals was in accordance with relevant institutional and ethical guidelines as approved for scientific study.
Preparation of polystyrene microplastics (PS-MPs)
Polystyrene microplastics (pristine polystyrene pellets and Styrofoam plate) were obtained from chemical market in Aba, Abia State, Nigeria, and crushed to obtain less than 5 mm particles which according to Rochman [20] is ingestible by organisms.
Experimental design
The animals were assigned to one of the seven groups, each with ten rats (5 male and 5 female, placed in separate cages). Group 1: served as the control and consisted of animals fed with standard rat feed and distilled water only. Group 2: consisted of rats fed standard rat feed incorporated with 1% polystyrene pellets (1% PSP). Group 3 consisted of rats fed standard rat feed incorporated with 5% polystyrene pellets (5% PSP). Group 4 rats received standard rat feed containing 10% of polystyrene pellets (10% PSP). Groups 5, 6 and 7 consisted of rats maintained on standard rat feed containing 1, 5 and 10% of Styrofoam plate particles (1% FP, 5% FP and 10% FP), respectively. The treatment was daily and lasted for 90 days.
Sample collection
At the end of treatments, rats were fasted overnight and sacrificed under anesthesia in slight diethyl ether. The blood samples were collected into EDTA and plain bottles before analysis.
Plasma preparation
The blood samples collected into EDTA bottles were spun at 3000 rpm for 10 minutes using a refrigerated centrifuge (Anke TDL-5000B, Shanghai, China) to obtain the plasma, which was subsequently used for the biochemical analysis.
Biochemical assays
The biochemical indices were determined in rat plasma using a UV/Vis spectrophotometer (Jenway, Staffordshire, United Kingdom) where applicable. The levels of rat plasma total cholesterol (TC), triglyceride (TAG), high-density lipoprotein cholesterol (HDL-C), and low- density lipoprotein cholesterol (LDL-C) was determined using Randox assay kits following the manufacturer’s protocol. Standard methods were used to determine the plasma concentrations of reduced glutathione, GSH [21], malondialdehyde, MDA [22] and the activities of catalase, CAT [23], glutathione peroxidase, GPx [24] glutathione s-transferase GST and superoxide oxide, SOD [25].
Atherogenic (lipid) indices
Atherogenic ratios were calculated from the lipid profile parameters.
Statistical analysis
All data were subjected to statistical analysis. Values were reported as mean±standard deviation (SD), while One-Way ANOVA was used to test for significance using statistical product service solution (SPSS). The results were considered significant at values (p) less than 0.05, which is 95% confidence level (p<0.05).
Results
Lipid profile and atherogenic indices in male and female rats
The determination of lipid profile showed that oral exposure of female rats to PS-MPs did not lead to significant (p>0.05) increase in plasma TC, TG and HDL levels compared to control. When compared to the normal control, the lipid profile of the female rats exposed to the polystyrene pellets and Styrofoam plates showed no observable difference except for LDL cholesterol. The LDL cholesterol was significantly (p<0.05) increased in female rats exposed to 1% PSP, 5% PSP and 5% FP compared to control (Figure 1). However, there was no significant difference (p<0.05) in the LDL cholesterol levels between the groups that received 5 and 10% microplastics.
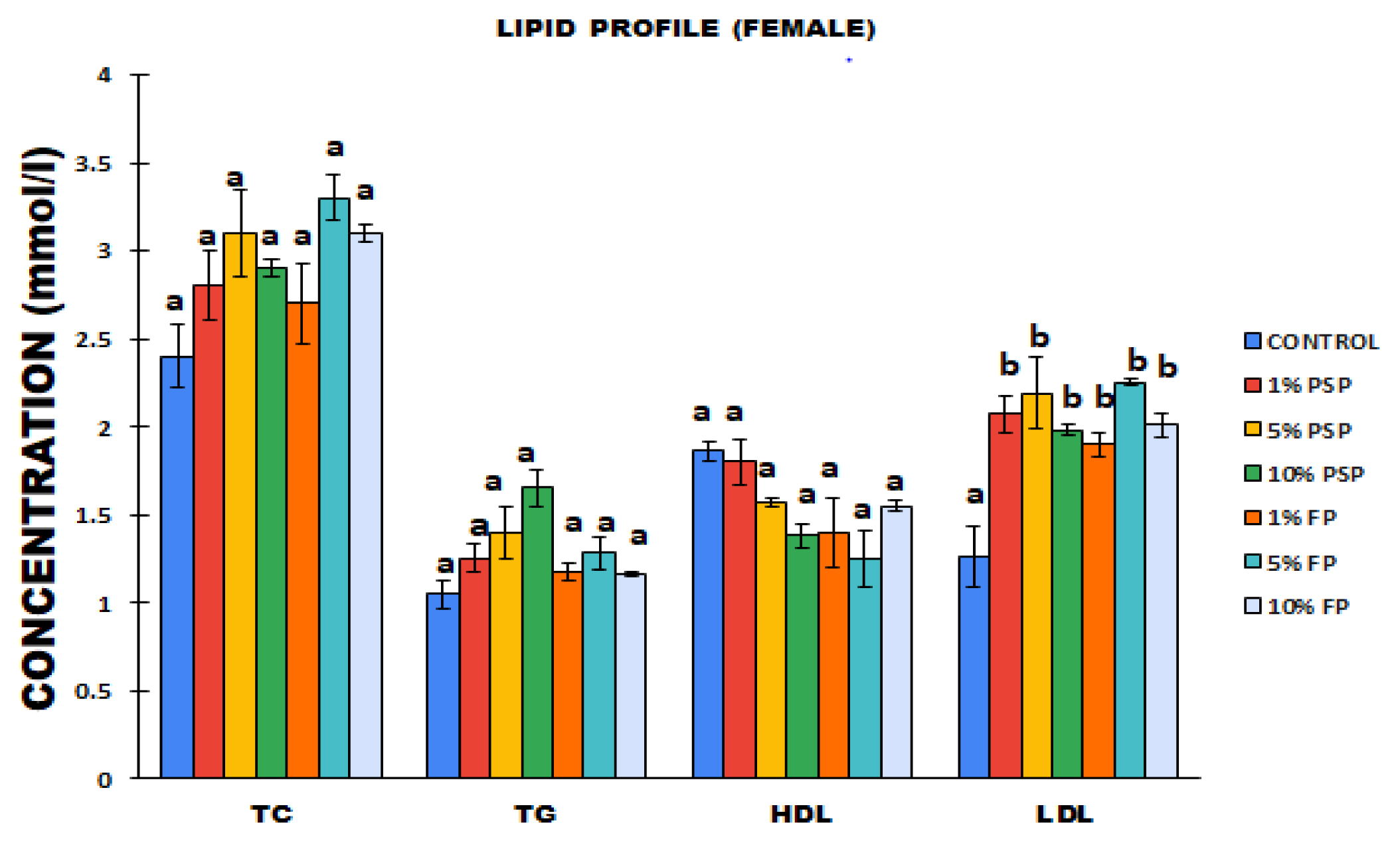
Levels of total cholesterol (TC), triglyceride (TG), high density lipoprotein (HDL) and low density lipoprotein (LDL) of female Wistar rats following exposure to polystyrene microplastics. Values are Mean± SD, n=5.
*Values with different subscript are significantly different at p<0.05. PSP=Polystyrene pellets, FP=Styrofoam plate.
The atherogenic indices of the microplastics exposed female rats showed negative AIP values (Figure 2). The 5% FP rat group had the highest AC, CRI-I, CRI-II, and non-HDL levels among the female rats. AC, CRI-I, CRI-II, and non-HDL levels were significantly increased in the microplastics exposed groups compared to the control. The atherogenic indices increase observed in the female rats that received PSP and FP were not proportionally dependent. For instance, the group that received 5% FP had higher AC, CRI-1, CRI-II, and non-HDL levels when compared to the 10% FP group.
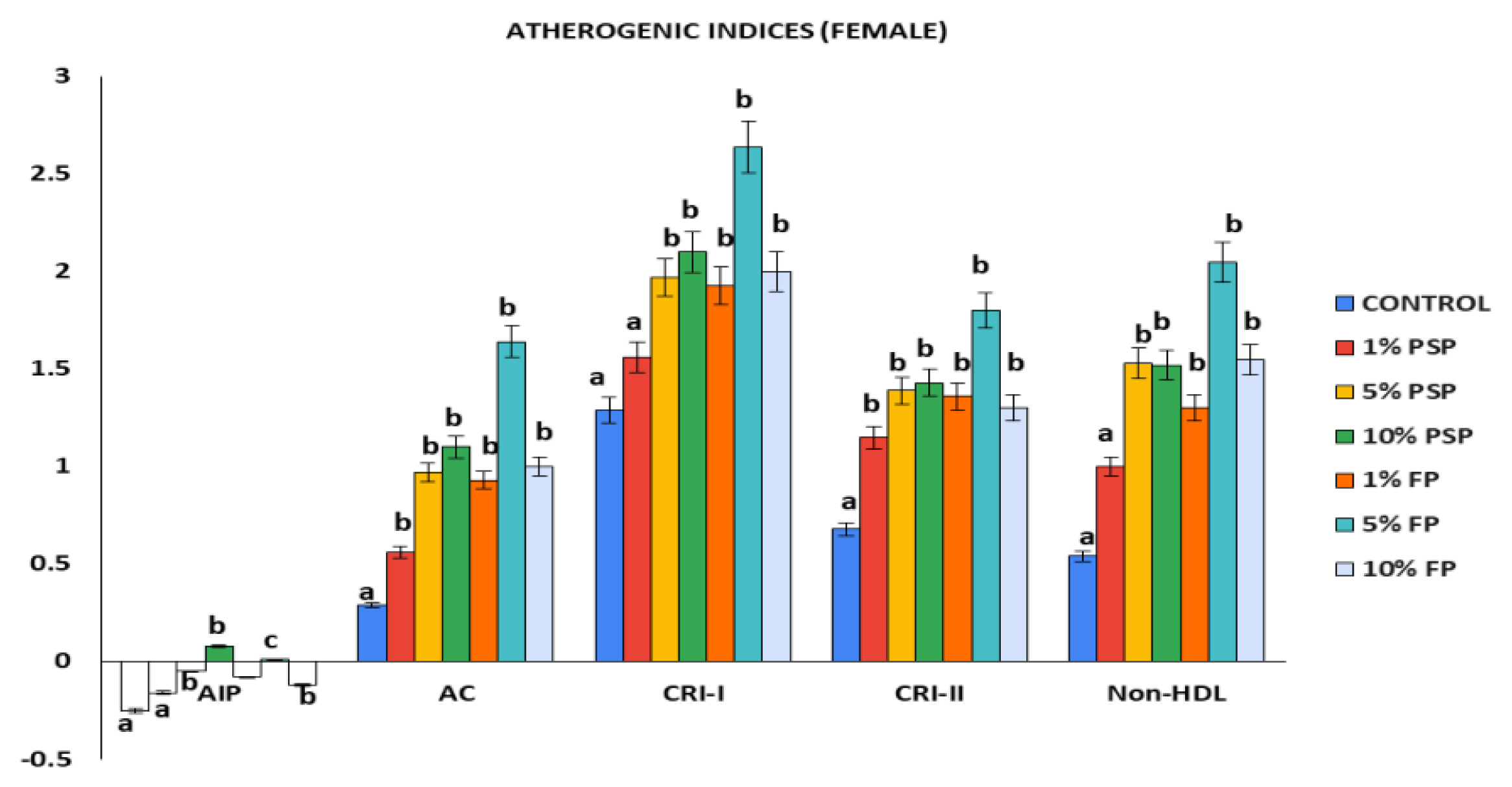
Atherogenic indices of female Wistar rats following exposure to polystyrene microplastics. Values are Mean±SD, n=5. *Values with different superscript are statistically significant at (p<0.05). PSP=Polystyrene pellets, FP=Styrofoam plate.
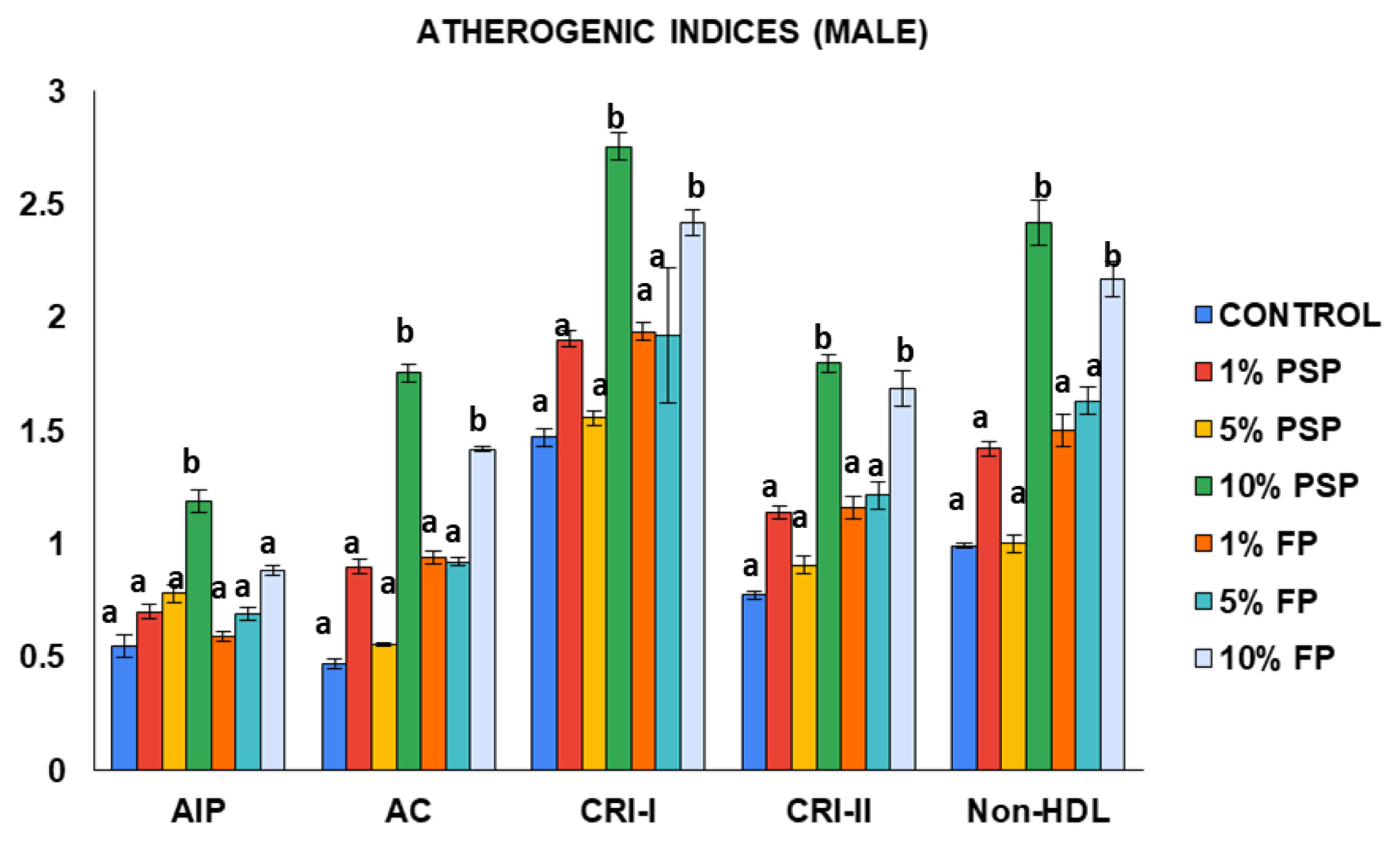
Figure 3 showed significant (p<0.05) increase in TC of male rats exposed to 5% FP compared to control. The measured plasma level of TG in the male rats from all exposure groups exhibited non-significant (p>0.05) increase when compared to the control. Additionally, HDL showed significant (p<0.05) decrease in male rats exposed to 1% PSP, 1% FP and 10% FP compared to control. On the other hand, plasma LDL increased with the proportion of microplastics in the feed, but significantly (p<0.05) only for the groups receiving 5% PSP and FP respectively.

Levels of total cholesterol (TC), triglyceride (TG), high density lipoprotein (HDL) and low density lipoprotein (LDL) of male Wistar rat following exposure to polystyrene microplastics. Values are Mean±SD, n=5. *Values with different superscript are statistically significant at (p<0.05). PSP=Polystyrene pellets, FP=Styrofoam plate.
The data from the atherogenic indices revealed that the 10% PSP exposed rat group had the highest occurring levels of AC, CRI-I, CRI-II, and non-HDL when compared to the other groups (Figure 4). The AIP, AC, CRI-I, CRI-II, and non-HDL levels in the male rats increased with the proportion of microplastics in the feed with. The male rats exposed to 10% PSP had higher atherogenic indices when compared to the group that received 10% FP. Similarly, to the female rat group, atherogenic indices were generally higher in the microplastics exposed groups compared to the control.
Oxidative stress status in male and female rats
After exposure to 1%, 5% and 10% FP and PSP, the redox parameters glutathione (GSH), glutathione peroxidase (GPX), glutathione transferase (GST), superoxide dismutase (SOD) and malondialdehyde (MDA) were not significantly altered (p>0.05) in all female rat treatment groups when compared to the control (Figure 5). Similarly, Figure 6 showed that the oxidative stress markers; glutathione (GSH), glutathione peroxidase (GPX), glutathione transferase (GST), superoxide dismutase (SOD) and malondialdehyde (MDA) experienced no significant (p>0.05) change in all male exposure groups when compared to the control.
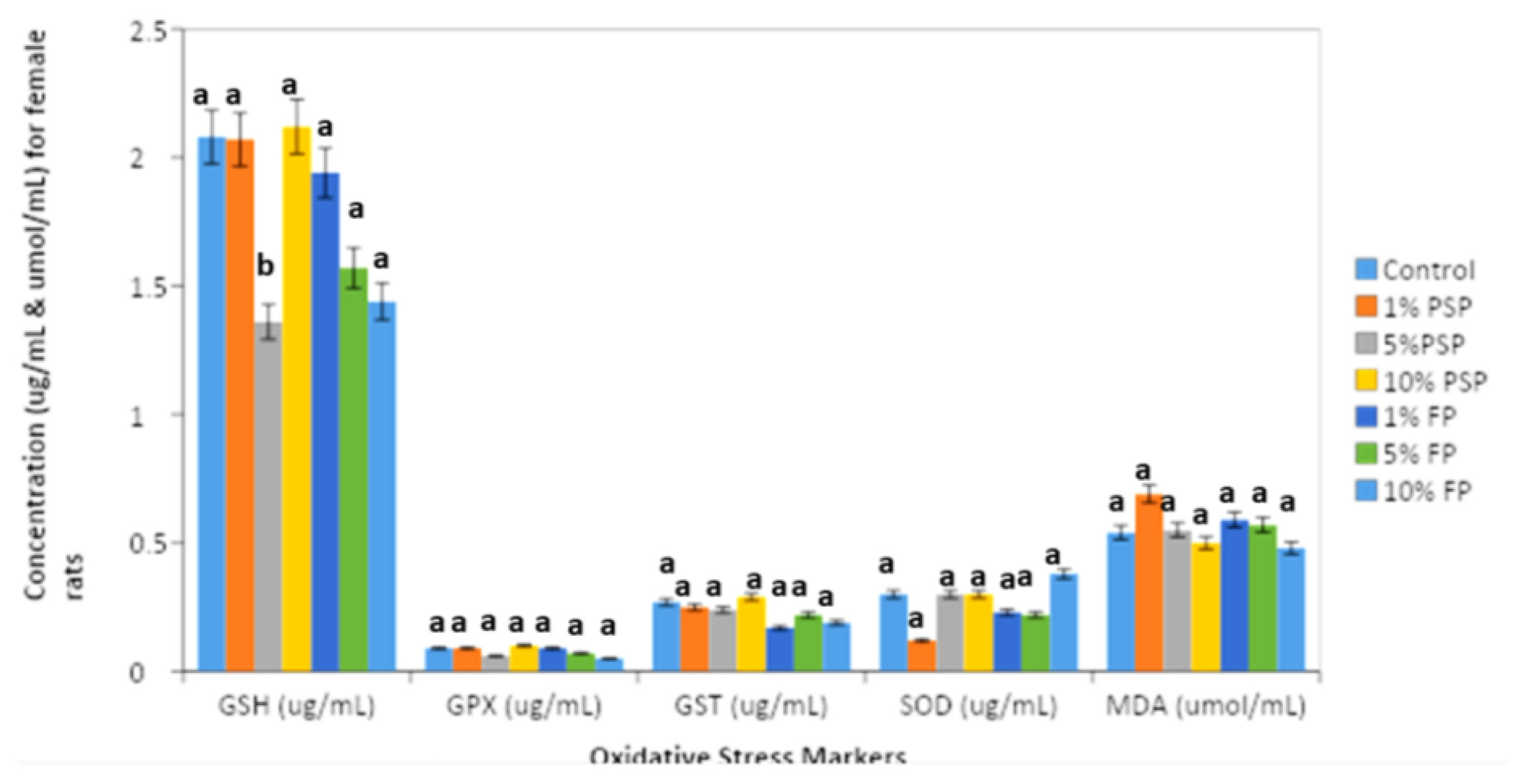
Levels of glutathione (GSH), glutathione peroxidase (GPX), glutathione s-transferase (GST), superoxide dismutase (SOD) and malondialdehyde (MDA) of female Wistar rats after polystyrene microplastics exposure. Values are Mean±SD, n=5. *Values are significantly different from the normal control group at (p<0.05).
Discussion
Dietary exposure to microplastics has become a growing public health challenge owing to the increasing problem of accumulation of microplastics in the environment. Also, anthropogenic microplastic particles from food packaging materials have become increasingly important as they are directly ingested by humans [29]. The present study reveals the impact of varying levels of microplastics derived from polystyrene food packaging materials on male and female rat lipid profile and oxidative stress status. This is coming from the background that previous studies have demonstrated the toxic potentials of micro and nanoplastics [30,31].
In the present study, the lipid profile data indicated that FP and PSP exposure led to elevated cholesterol, triglyceride and low density lipoprotein levels in both male and female rats. Dyslipidemia profile can be induced by environmental toxicants and is usually characterized by increased cholesterol, triglycerides, low density lipoprotein (LDL) and decreased high density lipoprotein [32]. Deng et al. [13] attributed the changes in cholesterol and triglycerides levels observed in mice to microplastics induced alterations in the serum levels of metabolites involved in lipid metabolism such as taurine, ethanol and choline. There are similarities between the attitudes expressed by HDL and total cholesterol levels in the rats in this study and those described by Amereh et al. [33]. They reported that different concentrations of nanoplastics significantly reduced the HDL cholesterol levels in rats. The AIP values of the male rats showed remarkable increase in comparison with the female rats, thus suggesting they were at a higher risk of developing coronary heart disease. AIP is generally used to assess cardiovascular risk factors and predict acute coronary events. AIP values of −0.3 to 0.1 are considered to be low risk while 0.10–0.24 and above 0.24 are associated with medium and high cardiovascular risk respectively [34]. To further assess cardiovascular risk following microplastics exposure in the rats, the cutoff level of CRI-I, CRI-II and AC were taken as > 4, >3 and >2 respectively for high risk [35]. The microplastics exposed female rat groups had lower cardiovascular risk levels as their CRI-I, CRI-II and AC values were below the cutoff level. In contrast, the 10% FP male rat group had borderline atherogenic index values. Bhardwaj et al. [36] reported that evaluating lipid ratios increases the chances of identifying at risk individuals especially in situations where lipid profile levels are below target range. This present study demonstrated that the same exposure levels of microplastics can induce varying degrees of response in male and female animals. However, further research will be needed to elucidate the mechanism of toxicity. Overall, it seems that the exposure of MPs may induce more cardiovascular risk in males.
Chemicals in the environment, such as microplastics (MP), can cause oxidative stress, resulting in the production of free radicals, changes in antioxidants and the oxygen free radical (OFR) scavenging enzyme system [37]. Polystyrene has been shown to cause oxidative stress and lipid peroxidation (LPO) in mice [38]. As a result, lipid peroxidation was utilized as a measure of oxidative stress in this study. Results showed that dietary exposure of rats to polystyrene MPs has no effect on the oxidative stress of Wistar rats hence no significant change in the malondialdehyde (MDA) level which was in contrast with the previous finding of Deng et al. [38], that polystyrene induced oxidative stress on mammals. Also, GSH, GPX, GST and SOD experienced no significant change in the MP exposure groups compared to the control indicating the antioxidant enzyme system was not disrupted following exposure in the current study. Yasin et al. [39] showed that MDA levels were elevated when rats were orally administered polystyrene nanoparticles for 5 weeks while Ilechukwu et al. [40] reported no significant change in GSH, GPX and MDA levels of male rats that were exposed to 10% microplastics. One possible explanation for the observed trend of no relative change in oxidative stress markers following PSP and FP exposure could be that the bioavailability of the 1–10% microplastic dosage used in the present study was not high enough to cause oxidative damage in the male and female rats. Walczak et al. [41] in their study earlier reported that the bioavailability of polystyrene particles can range from 0.2 to 1.7% in vivo. Overall, bioavailability remains a crucial factor in considering the potential toxicity of dietary exposure to microplastics. Hence, future toxicity studies based on larger MP dosages and longer exposure durations are recommended.
Conclusions
This study provides insight into the role of MPs as potential agents of dyslipidemia and oxidative imbalance in mammals. In the dose range examined, PS-MP produced no observable oxidative stress in all treatment groups. In contrast, both the polystyrene pellets and polystyrene foam showed dyslipidemia behavior in the male and female rats at the dose range studied with the atherogenic indices indicating higher cardiovascular risks in the male rats. Further investigations on the dietary exposure of microplastics as risk factors for development of atherosclerosis in mammals is required.
Notes
Authors declare no conflict of interest.
CRediT author statement
UCN: Conceptualization, Investigation, Resources; CJO: Conceptualization, Investigation, Supervision, Formal analysis, Writing-Reviewing & Editing; II: Investigation, Methodology, Validation; CJO: Methodology, Writing-Reviewing & Editing; DCB: Methodology, Supervision, Validation.