Chronic toxic effects of polystyrene microplastics on reproductive parameters of male rats
Article information
Abstract
Microplastics have become a significant environmental concern. However, information on toxicity of microplastics in terrestrial organisms is limited. In this study, the chronic toxic effects of polystyrene microplastics (PS-MPs) on the reproductive system and serum antioxidants of male albino Wistar rats fed for 90 days with standard rat feed containing 1–10% granules of crushed polystyrene disposable plates were evaluated. Significant reductions in volume, motility, epididymal sperm count and serum testosterone level were observed. Histological examination of testicular architecture showed distorted testes with vacuolated seminiferous tubules at the highest percentage, together with increased catalase and decreased superoxide dismutase activities. This study showed that ingestion of PS-MPs caused reproductive dysfunction in male rats and contributes to understanding the potential toxicity of microplastics in terrestrial animals.
Introduction
Microplastic pollution has gained global attention in recent times due to its environmental ubiquity. They are plastic particles of less than 5 mm in any one dimension [1,2]. Microplastics are of two main types; primary microplastics which are manufactured as such, and secondary microplastics which are degradation fragments of larger pieces [3]. They arise from littering, storm water overflow, wastewater treatment plants, biosolid application, atmospheric deposition, release from industries, and poor waste disposal [4–6].
Microplastics occur in different environmental compartments [7–10]. As mixtures of various chemicals and materials, microplastics may cause a multitude of harmful effects [11], such as physical blockage of the digestive tract, leakage of plastic additives, and release of adsorbed contaminants [12,13]. Organisms ranging from aquatic invertebrates to mammals may ingest microplastics [14–17]. Accumulation of microplastics in the digestive system and transfer to other tissues through the circulatory system in mice as well as the trophic transfer of microplastics from aquatic to terrestrial mammals has been reported [18,19]. Adverse effects of exposure include disturbance of lipid metabolism, oxidative stress, decreased reproductive output, immobilization and triggered molting in copepod [20–23]. Polystyrene is a major constituent of microplastics reported in the environment [1,8,9], due to its mass production and widespread use for insulation [24]. Polystyrene absorbs persistent organic pollutants and polycyclic aromatic hydrocarbons more extensively than other plastic polymers [25]. When used as plates, cups, and food packaging materials, food contact chemicals may transfer into food and be ingested by humans [26,27], and they are prone to weathering and resistant to biodegradation [24,28]. Currently, there are few studies on the adverse effects of microplastics on terrestrial mammals, as most studies are focused on aquatic organisms despite the ubiquitous presence of microplastics in the terrestrial environment [29,30]. Further, the majority of toxicological studies have been conducted with virgin or pristine microplastic particles. In this study, granules from disposable polystyrene plates were evaluated regarding their effects on the reproductive and antioxidant system of male Wistar rats.
Materials and Methods
Microplastic feed preparation
Packs of polystyrene disposable plates purchased from a local store were thoroughly washed with distilled and deionized water and dried at room temperature for 48 hours. The plates were crushed with a manual blender and particles that passed through a 2 mm mesh sieve were mixed into standard rat feed (Ladokun Feeds, Ibadan, Nigeria) in a stainless-steel mixing bowls at 1%, (10 g polystyrene particles in 1 kg of feed), 5% (50 g polystyrene particles in 1 kg of feed), and 10% (100 g polystyrene particles in 1 kg of feed).
Animal handling and experimental design
Twenty-four male albino rats obtained from the animal house of the University of Benin, Nigeria, weighing between 106–125 g were acclimatized for two weeks under standard laboratory conditions and distributed into four groups of six rats each in metal cages (56×30×22 cm). Group 1 served as control and received water and normal feed ad libitum. Groups 2, 3 and 4 received feed containing crushed polystyrene at 1%, 5%, and 10% respectively, which were constituted in 150 g of feed and fed to treated rats daily in the first 30 days. It was increased to 300 g daily for the next 60 days. Treatment was done such that the animals were fasted overnight after which the rats in the treatment groups were made to first eat all the polystyrene treated feeds before the normal feed. On day 90, the animals were sacrificed after anesthesia using chloroform before collection of seminal fluid. Blood collected from each animal via cardiac puncture with a 5 mL syringe was transferred into centrifuge tubes and centrifuged at 3000 rpm for 15 minutes. The serum obtained was used for determination of testosterone, glutathione (GSH), and malondialdehyde (MDA), as well as the activities of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px).
Organ collection and histology
The rats were dissected immediately after sacrifice and the testes were removed, cleared of adherent tissues and weighed on an electronic scale. The organs were fixed in Bouin’s solution for six hours before transfer to 10% formalin for 24 hours. Testes samples were processed routinely and embedded in paraffin blocks. Slides were prepared, stained with hematoxylin and eosin for histological evaluation under a light microscope. Microphotographs of the slides were also taken.
Serum analyses
Total serum testosterone was determined using an ELISA kit following the manufacturer’s protocol (Diametra, Perugia, Italy). Standard methods were used to determine the serum concentrations of GSH [31], MDA [32] and the activities of CAT [33], GSH-Px [34] and SOD [35]. Briefly GSH was measured by analyzing the thiol complexes reduction of DTNB (5, 5′-dithiobis (2-nitrobenzoic acid)) and absorbance read at 412 nm. Trichloroacetic acid (2.5%) was used to remove interfering proteins. Thiobarbituric acid method was used for the determination of MDA. Decrease in absorbance from the degradation of H2O2 by CAT enzyme in the presence of phosphate buffer was measured to determine CAT activity. Extinction coefficient of 39.4 mM−1 cm−1 was used for the calculation of H2O2 decomposition. One unit of activity is equivalent to 1 mM of H2O2 degraded per minute. GPx activity was determined by oxidation of NADPH to NADP+. The decrease in absorbance which is proportional to the GPx activity was measured at 340 nm. SOD was determined by inhibition of nitro blue tetrazolium (NBT) reduction. The amount of enzyme that leads to 50% inhibition of NBT reduction expresses one unit of enzyme activity. NBT reduction was measured at 600 nm.
Sperm motility and count assay
Sperm motility was determined according to Zemjanis [36]. The microscopic fields were viewed under a light microscope to determine motile and non-motile spermatozoa. Sperms were counted according to Pant and Srivastava [37], employing the improved Neubauer hemocytometer (depth 0.1 mm, LABART, Waldbüttelbrunn, Germany).
Morphology and viability assay
Morphology and viability were determined from 400 spermatozoa counted in smears stained according to Wells and Awa [38], stains (0.2 g eosin and 0.6 g fast green dissolved in distilled water and ethanol in 2:1 ratio) [39].
Statistical analysis
All data were analyzed using IBM SPSS Statistics version 21 and presented as mean±standard error of mean and analyzed by one-way analysis of variance followed by multiple comparison test. Significance of the mean differences was considered at p<0.05.
Results and Discussion
There was a decrease in the body weight of the treated groups which was significant (p<0.05) for Groups 3 and 4 (Table 1). Significant decrease in body weight of mice after six weeks exposure to polystyrene microplastics has been reported by Xie et al. [40]. Similar reduction also observed in this research may be due to the difficulty in expelling larger sized MPs from the body, resulting to heavy burden on gastro-intestinal tract which may reduce food absorption and cause decreased body weight [41].
Reproductive parameters
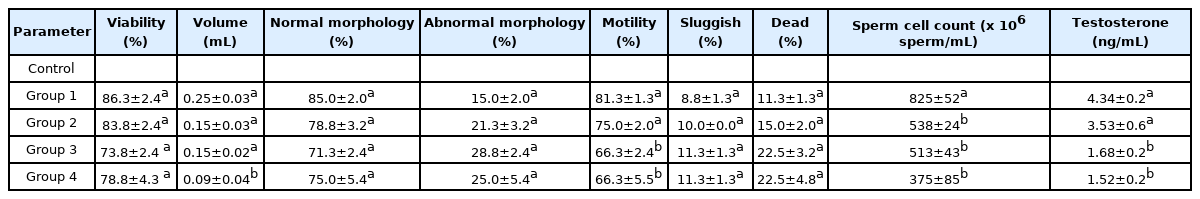
The sperm of all rats was milky in appearance while the viscosity and pH were normal and 8.0 respectively. The effects of the polystyrene microparticles on the sperm quality and testosterone levels of the male Wistar rats are shown in Table 2. There was a non-significant reduction in viability and morphology of the sperm cells and a non-significant increase in abnormal morphology and dead cells. The semen volume of the exposed group also decreased but significant only for Group 4 that received the highest proportion of microplastics. Sperm motility also decreased across the treated groups, significant (p<0.05) for Groups 3 and 4. The sperm cell count showed a significant decrease (p<0.05) for all treated groups. Testosterone also significantly decreased for Groups 3 and 4. This result indicates that polystyrene granules from polystyrene plates have adverse effects on the reproductive capabilities of male Wistar rats. It also agrees with other studies which found that polystyrene microplastics adversely affected the reproductive capacity of oysters and marine medaka [40–42].
Histology
The histology of the testes (Figure 1) showed marked difference between the control group and the treated group. Photomicrograph of the control group (GP 1) shows seminiferous tubules containing spermatogenic cells, spermatozoa, and Sertoli cells, while the photomicrograph of the groups that received polystyrene (GP 2–4) shows morphological abnormalities in progressive distortion of testes that became prominent with vacuolation of the seminiferous tubules and reduced sperm cell levels in the group that received the highest proportion of microplastics (GP 4). Spermatogenic cells became loosely arranged in the treated groups with blank cavities appearing on the tissues with increasing PS-MPs [40].
Antioxidant assay
Both GSH-Px and GSH biomarkers showed no particular trend in their response to the exposure to polystyrene microplastics (Table 3). MDA increased marginally in the group that received the polystyrene microplastics but not proportionally dependent. The increase was not significant (p<0.05) except for the group that received 5% microplastics (Group 3), in contrast to the significant increase in MDA upon exposure to polystyrene microplastics in marine medaka [42,43]. CAT increased with the proportion of microplastics in the feed, but significantly (p<0.05) only for the group receiving the highest proportion (Group 4). SOD facilitates the breakdown of superoxide radical into molecular oxygen or hydrogen peroxide [44]. There was a significant decrease (p<0.05) in the SOD activity of the exposed groups except for the group receiving 1% microplastics. Similar trend for CAT and SOD activity in earthworms exposed to soil treated with microplastics has been reported [17].
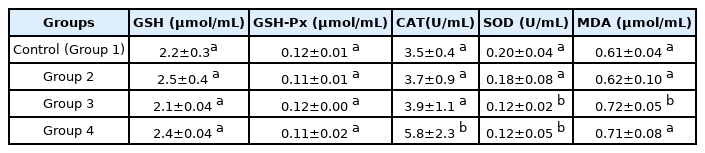
Effect of polystyrene microparticles on the oxidative stress/antioxidant status of male Wistar rats.
These antioxidant enzymes are markers to assess early oxidative damages caused by xenobiotics. Increased GSH-Px, SOD, and decreased CAT activity were observed in mice exposed to microplastics [19]. Disturbed antioxidant system in aquatic organisms after exposure to microplastics has also been reported [40,45,46]. These results indicate that exposure of Wistar rats to polystyrene microplastics induced responses that caused imbalance in their antioxidant defense system.
In examining the factors that contribute to microplastic-induced toxicity, Choi et al. [47] reported that smaller microplastic size and long exposure time increased intracellular levels of reactive oxygen species. Smaller particles have been reported to induce more response due to large surface area that enhances their bioavailability [48–50]. These two factors; chronic exposure and the smaller components of the polystyrene microplastics may have contributed significantly to the adverse effects observed in this study.
Conclusions
Findings of this study suggest that under environmentally relevant conditions, polystyrene microplastics may cause adverse effect on the reproductive and antioxidant system of male Wistar rats. Knowledge gained from this study can be applied to the study of effects of microplastics in terrestrial animals.
Notes
Authors declare no conflict of interest.
CRediT author statement
II: Conceptualization, Investigation, Supervision, Writing-Original draft preparation; BEE: Investigation, Supervision, Formal analysis, Writing-Reviewing and Editing; IOB: Investigation, Writing-Reviewing and Editing; CJO: Methodology, Validation; OSO: Investigation, Methodology, Resources; UEM: Methodology, Writing-Reviewing and Editing; CEI: Conception, Methodology, Writing-Reviewing and Editing; NJO: Methodology, Writing-Reviewing and Editing, Validation.