Protective action of polysaccharides from Laurencia papillose (Rhodophyta) against imidacloprid induced genotoxicity and oxidative stress in male albino rats
Article information
Abstract
Imidacloprid (IMI), the main component of neonicotinoid insecticides, promotes oxidative stress and genotoxicity in mammals. The aim of this experiment is to assess oxidative stress in liver cells and genotoxicity of erythrocytes for rats exposed to sub-lethal doses of IMI and the protective effects for Rhodophyta as antioxidant material versus imidacloprid. A total of 30 adult male albino rats (average body weight, 190–200 g) were divided into six groups (n=5) as follows: group 1 served as the control, group 2 received 200 mg/kg red algae, group 3 received 45 mg/kg IMI (high-dose group), group 4 received 22.5 mg/kg IMI (low-dose group), group 5 received 200 mg/kg red algae +45 mg/kg IMI, and group 6 received 200 mg/kg red algae +22.5 mg/kg IMI. After 28 d of treatment, the antioxidant activity of the crude extract of red algae was assessed in terms of free radical scavenging activity and found to be higher in TCA (75.57%) followed by DPPH (50.08%) at concentration 100 μg extract and a significant increase in lipid peroxidation and reductions in glutathione were observed in liver cells were intoxicated with high and low doses of IMI. Moreover decreases in catalase and glutathione peroxidase parameters in same previous groups which indicated oxidative stress. In addition significant increases in micronucleus frequency (MN) in the bone marrow of the rats as a genotoxicity marker which indicated DNA damage in erythrocytes cells with alterations in the histopathology of liver cells were also noted such as necrosis, inflammatory cells, infiltration, and necrobiotic changes. Whereas Rhodophyta succeeded in alleviation the oxidative damage and genotoxicity induced by the insecticide. In conclusion, IMI demonstrates hazardous effects, such as alterations in antioxidant status and mutagenicity of erythrocytes and polysaccharides from Rhodophyta has good antioxidant activity in vivo model systems against imidacloprid.
Introduction
Imidacloprid (IMI) is the most widely used neonicotinoid insecticide in agriculture. Indeed, the global annual production of IMI became increasingly, for example, its production has reached to 23000 t in 2016 in China and the pesticide is used to treat 140 crops in over 120 countries [1–3]. The selective toxicity of IMI is due to its function as an agonist of the nicotinic acetylcholine receptor, which leads to the blockage of neuronal signaling, acetylcholine accumulation, and, finally, death [4].
Some studies have demonstrated the high oxidative potential of IMI [5,6]. Oxidative damage, which leads to free radical production, may occur following pesticide exposure [7]. Free radicals attack macromolecules, such as lipids and proteins [8], and cause distinct DNA alterations [9]. The mechanisms by which cells resist oxidative stress and repair damaged macromolecules could be divided into the enzymatic pathway, which involves catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), and glutathione peroxidase (GPx), and the non-enzymatic pathway, which involves glutathione (GSH); these mechanisms stimulate the scavenging of free radicals [10].
Neonicotinoid insecticides are widely used in the environment. Thus, the mutagenicity of IMI must be carefully evaluated. The mutagenic potential of IMI has been observed in in vitro and in vivo systems using cytogenetic endpoints, such as the micronucleation method [11,12]. A previous study indicated that exposure to 22 mg/kg IMI for 28 d could induce significant increases in micronucleus frequency (MN%) and chromosomal aberrations in mice [13].
Red algae (Rhodophyta) are commonly believed to have defensive or protective functions [14]. Marine algae contain large amounts of polysaccharides in their cell wall [15]. The polysaccharides of marine algal species make up 4%–76% of their dry weight. The major polysaccharides of red algae are carrageenans, agars, xylans, loridean starch, and water-soluble sulfated galactans [16]. The carrageenans of red algae have immuno-modulatory, anticoagulant, antithrombotic, antiviral and antitumor activities [17]. Carrageenans from gigartinaceae and tichocarpaceae show strong antioxidant activity against superoxide anions, OH radicals, NO, and H2O2 [18].
The dramatic increase in the use of IMI worldwide could lead to the contamination of drinking water and food, thereby exposing humans and animals to the risks associated with IMI. Recent evidence indicates that IMI produces reactive oxygen species (ROS), which could lead to oxidative stress and genotoxicity. Thus, considerable attention should be paid to the toxicity of IMI and the protective effects of natural compounds against oxidative damage and mutagenicity. The purpose of our study is to monitor the toxicity of IMI in in vivo models and determine the potential use of algal polysaccharides as a natural antioxidant against IMI toxicity causing liver injury.
Materials and Methods
Chemicals
IMI (purity, 95.45%) was gifted by the Pesticide Analysis Department, Central Agricultural Pesticides Laboratory, Dokki, Egypt. Fetal calf serum (FCS) was obtained from Boehringer Ingelheim GmbH (Germany). May–Grunwald and Giemsa stains were procured from LOBE CHEMIE Pvt. Ltd. All other chemicals used in this study were purchased from Sigma Chemical Co. (USA). All test solutions were freshly prepared before each experiment.
Collection and identification of marine alga
Rhodophyta was collected from Red Sea (Ain Al-Sokhna 2020). Algea was washed a few times with water at that point cleared out for air dryness. Crushed samples have been stored in glass containers at room temperature. It was identified by Professor Rauhaiya Abdul-Latif, Department of Botany, Faculty of Science, El-Azhar University, to whom authors are very indebted.
Extraction of polysaccharides
1 kg of algae was soaked in hot distilled water at room temperature for 2 h with shaking. The extract was filtered using Whatman No. 1 filter paper under reduced pressure. Precipitation of polysaccharides was carried out with absolute ethanol and centrifuged at 5,000 rpm for 20 min for pooling.
Total sugars of extracted algae
The total sugars content in polysaccharide extract was estimated color metrically by the phenol-sulfuric acid method using D-glucose as a standard [19].
Determination of DPPH and total antioxidant capacity (in vitro assay)
Measurement of algal polysaccharide as antioxidant agent in vitro DPPH free radical-scavenging assay. The ability of polysaccharides extracted from extract to scavenge DPPH. The determination of free radicals was done according to Ye et al. [20]. Different concentrations of samples were added to 0.1 mM ethanolic DPPH solution (3.0 mL). The absorbance was measured at 517 nm, after incubation for 30 min in dark. The scavenging percentage was calculated using the following equation:
One mL of a polysaccharide extract from algae and standard (ascorbic acid) (25 to 100 μg/mL) was blended with 3 mL of the following solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). Tubes were incubated at 95 °C for 90 min. The absorbance of each sample was measured at 695 nm, after cooling according to Prieto et al [21].
Animals
All animals (30 rats) were acclimatized for 2 w before commencing the experiment and were maintained under normal conditions as 55-50% humidity, 22±2 °C temperature and 12±1 h light-dark with feeding on a standard diet and free water. The animals were owned by National Research Centre. The animal protocols in this study were approved by the Animal Ethics Committee of Cairo University, which complies with the Guide for the Care and Use of Laboratory Animals and its associated guidelines (EU Directive 2010/63/EU) for animal experiments (Permission No. CU/III/F/5/20).
Animals treatment
IMI doses are selected based on published LD50 value, which is recorded to be 450 mg/kg body weight [22]. A period of experimental depends on the sub-acute study was conducted by OECD, Test No. 407 [23]. IMI was dissolved in corn oil and was administered by oral gavage daily consequently for 28 days plus the pretreatment 200 mg/kg at red algae as antioxidant protection dissolved in distilled water about half hour before intoxication [24]. The animals were divided at random into six groups with 5 animals in each group and were treated orally as follows:
Group 1: served as the control (received 5 mL/kg corn oil).
Group 2: served as algae alone (received red algae at dose 200 mg/kg).
Group 3: received IMI at a dose of 45 mg/kg body weight (BW) (1/10 LD50 high dose)
Group 4: received (IMI) at dose 22.5 mg/kg (BW) (1/20 LD50 low dose).
Group 5: received red algae at dose 200 mg/kg pretreatment plus high dose of IMI.
Group 6: received red algae at dose 200 mg/kg pretreatment plus low dose of IMI.
Biochemical parameters
The animals were sacrificed by cervical dislocation after 28 d. Livers were quickly removed, rinsed in iced saline, homogenized and used to determine LPO levels and GSH contents. The liver homogenates were centrifuged at 14000 rpm for 20 min at 4 °C, and the supernatant was collected for antioxidant enzyme activity (CAT, SOD, and GPx) measurements.
Lipid peroxidation level in rat liver
MDA formation as a marker of lipid peroxidation was determined spectrophotometrically at 532 nm based on its reaction with thiobarbituric acid (TBA) according to Nair and Turner [25].
Antioxidant enzyme activities in rat liver
Reductions in GSH content were measured concurring to [26]. CAT activity was assayed according to Beers and Sizer [27]. SOD activity was determined according to Nebot et al. [28]. Determination of GPx activity was performed according to Chiu et al. [29].
Micronucleus test in bone marrow
The frequency of micronucleated erythrocytes was measured according to the method developed by Schmid [30]. Male rats were sacrificed by cervical dislocation. The femoral bone marrow was flushed out using 1 mL of FCS and centrifuged at 2000 rpm for 10 min. The supernatant was discarded. Smears were prepared for each animal, fixed in methanol, and stained with 10% Giemsa and 50% May-Grunwald stains. The smears were screened at a magnification of 1000× by using a light microscope. The number of erythrocytes with one or more micronuclei in at least 1000 erythrocytes per animal was counted. MN% was calculated as follows:
Histopathological examination
Liver samples were taken from all groups and placed in 10% formol saline for 24 h. The samples were embedded in paraffin wax, cut into 4 mm sections, and then stained with hematoxylin and eosin. The slides were studied under a microscope to evaluate pathomorphological changes in the liver tissues [31].
Statistical analysis
The data are expressed as mean±standard error. The groups were compared by one-way analysis of variance by using the Statistical Package for Social Sciences (SPSS, SPSS Inc., Chicago, IL, USA) version 15.0 p<0.05 indicated statistical significance.
Results and Discussion
Characteristics of algal polysaccharides
The polysaccharide yield of Rhodophyta was 7% (dry weight), and total sugars in the polysaccharide extract averaged 90% (Table 1). Many red algal polysaccharides have good antioxidant activity in vitro and in vivo. These polysaccharides are known to inhibit the damage caused by oxidative stress in various organs of the digestive tract and the liver [32].
Determination of DPPH and total antioxidant capacity (in vitro assay)
The total antioxidant activity of the polysaccharide extract was assessed in terms of the ability of the latter to reduce phosphomolybdic acid (Table 2). The antioxidant activity of the extract increased with increasing concentration of the polysaccharides. Red algal polysaccharide demonstrated antioxidant activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals and good reducing power. Polysaccharides could enhance the production of antioxidant enzymes, such as CAT, SOD, and GPx, by regulating the expression of genes encoding these enzymes and prevent cell death by regulating apoptosis factors, such as Caspase-9, Bax, and JNK, to repair the damage caused by oxidative stress [33]. Jaballi et al. found that red algae are effective antioxidants against Maneb, a fungicide that causes hepatotoxicity, genotoxicity, and oxidative stress in the blood and bone of adult rats when treated daily for 7 d [34].
Measurement of glutathione concentrations
The concentrations of pesticides and their structures could induce changes in the efficiency of the GSH redox cycle [35]. GSH, the most abundant non-protein thiol in toxic compounds, maintains the thiol/disulfide status and offers protection against toxic substances and oxidative damage [36]. Compared with the control, IMI treatment induced statistically significant decreases in GSH levels at all doses tested (p<0.05). A significant increase in GSH level in the high-dose IMI+algal treatment group (22.30±1.19) when compared with the high-dose IMI group (16.79±1.16) was also noted. This finding indicates that the algae could reduce the toxicity of the pesticide. No significant difference between the low-dose IMI and low-dose IMI+algal treatment groups was observed after 28 d of exposure to IMI (Table 3). This finding may be attributed to the accumulation of IMI metabolites in the liver, which is the first organ for any detoxification pathway. Metabolite accumulation may produce excessive free radicals/ROS in tissues. ROS could lead to the failure of the membrane structures of the mitochondria, which undergo permeability changes following enhanced lipid peroxidation (LPO) and GSH depletion [9]. IMI may trigger apoptosis by modulating various signaling pathways, such as GSH depletion [37]. Bal et al. demonstrated that treatment of rats with 8 mg/kg IMI daily for 3 months causes decreases in GSH compared with that in the control group [38]. Lonare et al. recorded significant increases in LPO (p<0.05 ) and reductions in GSH (p<0.05) in male rats [39].
Evaluation of tissue lipid peroxidation
LPO occurs via the disruption of polyunsaturated fatty acids, which could be used as a biomarker of oxidative damage and measured in terms of changes in malondialdehyde (MDA) [40]. Pesticides can promote oxidative stress through free radical-induced LPO, which changes membrane fluidity and DNA structures and promotes carcinogenic effects [41,42]. We observed that IMI treatment causes significant increases in MDA content when compared with the control in all dose groups, except in the low-dose IMI+algal treatment group, after 28 days of exposure. Treatment with 200 mg/kg algal polysaccharides and 45 mg/kg IMI did not reduce the effect of oxidation. However, the low IMI dose+algal polysaccharide group showed less lipid oxidation when compared with the negative and positive control groups (p<0.05; Table 3). This finding may be attributed to the presence of phenolic compounds, which inhibit intracellular ROS formation, in the algae. Kang et al. demonstrated that marine algae could inhibit the LPO induced by phenolics when treated AAPH in zebrafish compounds [43]. Exposure to IMI may produce ROS, which can also oxidize membrane phospholipids, leading to the deterioration of the membrane structures of the mitochondria and other cytoplasmic components [44]. The metabolism of IMI produces nicotine compounds, which could generate large amounts of reactive free radicals as a consequence of the induction of cytochrome CYP2A6 in the growth of lipid [45]. Yardimci et al. observed high oxidative toxicity and a significant increase in thiobarbituric acid (TBA) reactive substances in the kidneys of male rats after IMI exposure [46].
Antioxidant enzyme activity in the liver tissue
Enzymes such as SOD and CAT remove the ROS produced during the detoxification of xenobiotics [47]. Decreases in antioxidant parameters (e.g., GST, GR, GPx, SOD, and CAT) responsible for the capture of free radicals generated during exposure to chemicals induces oxidative stress, which could explain the increased MDA observed in tissues [48]. Our results revealed that IMI treatment produces statistically significant decreases in CAT and GPx when compared with the control group (p<0.05) and that algae could resist the toxicity of low-dose IMI and normalize CAT and GPx activity. Non-significant in SOD were observed at all IMI doses administered when compared with the control group (p<0.05) however, treatment with algal polysaccharides in the low-dose IMI group improved the activity of this enzyme Table 3. Three reasons may explain the decline in CAT activity noted in this work. First, pesticide exposure produces OH radicals, which leads to a decrease in CAT activity [49]. Second reason, increment of concentration of free radicals or depletion of reduced glutathione a substrate for different antioxidant enzymes like GPx [48]. Third, inhibition of CAT activity has been related to the binding of toxic substances to the-SH groups of enzyme and increased H2O2 and/or superoxide radicals [50]. Our findings indicate that algal polysaccharides could improve CAT levels because marine algae possess many phytochemicals with different bioactivities, including antioxidant, anti-inflammatory and anticancer properties [14,51]. El-Gendy et al. and Lonare et al. recorded significant decreases in CAT, SOD, GPx, and GST activity when IMI is orally administered to rats at different doses [51,52].
Micronucleus frequency in bone marrow cells
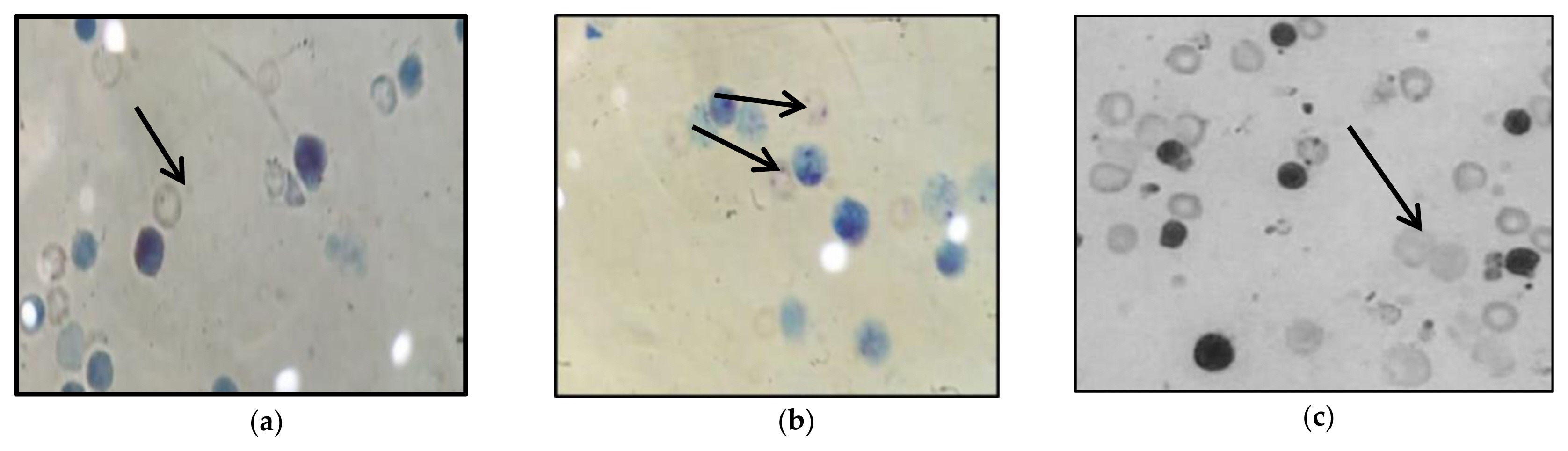
MN% in the bone marrow of rats was used to evaluate the mutagenesis of IMI in erythrocytes. Table 4 and Figure 1 summarizes the effects of IMI on the MN% of rat bone marrow cells after 28 d of exposure. It was induced increase in micronucleus frequency and significantly increased the micronucleus frequency between all doses with algae or without algae when compared to the control (p<0.05). Algal polysaccharides did not any ameliorate the DNA damage induced by the toxicity of IMI. IMI is an alkylating material that can induce clastogenesis through cellular DNA damage. Thus, the presence of an electronegative group (nitroimineN-substituent¼NNO2) in the nitroguanidine moiety of neonicotinoid insecticides could bind IMI to DNA, leading to the induction of breaks [53]. The DNA damage induced by IMI may also be attributed to direct interactions between the pesticide and the tested compounds and/or their metabolites, which destabilize the DNA structure and lead to DNA breaks [54]. Arslan et al. suggested that IMI metabolites may directly bind to DNA. The substance is metabolized more effectively in male rats than in female rats, leading to the accumulation of IMI metabolites [55]. Alterations in the GSH redox cycle by toxic materials cause oxidative stress, which leads to mammalian genotoxicity [56]. In agreement with our results, Karabay and Oguz recorded the potential cytogeneticity of IMI in vivo [12]. Bagri et al. demonstrated the mutagenicity of IMI in male mice when treated at a dosage of 22 mg/kg orally for 28 days [13].

Incidence of micronucleated erythrocytes in the bone marrow in albino rats treated with imidacloprid alone with imidacloprid plus Rhodophyta after 28 d administration orally.
Histopathological findings
Figure 2 shows the histopathology of all treatments. The control and algal polysaccharide groups have normal liver histological structures Figure 2, A and I. The liver of the rat administrated 45 mg/kg IMI showed centrilobular necrosis surrounding the central vein, as well as degenerative and necrobiotic changes in the portal vein C (Figure 2, B and C). Treatment with 22.5 mg/kg IMI induced necrosis, with inflammatory cells, infiltration into the portal area, vascular degeneration, necrobiotic changes, and liver necrosis Figure 2, D and E. The parenchyma of the hepatocytes showed vascular degeneration, necrobiotic changes, necrosis, and inflammation-associated cell infiltration after treatment with 45 mg/kg algal polysaccharides (Figure 2, F and G). Treatment with IMI at a dose of 22.5 mg/kg under the protection of the algal polysaccharides noticed no histopathological alterations.
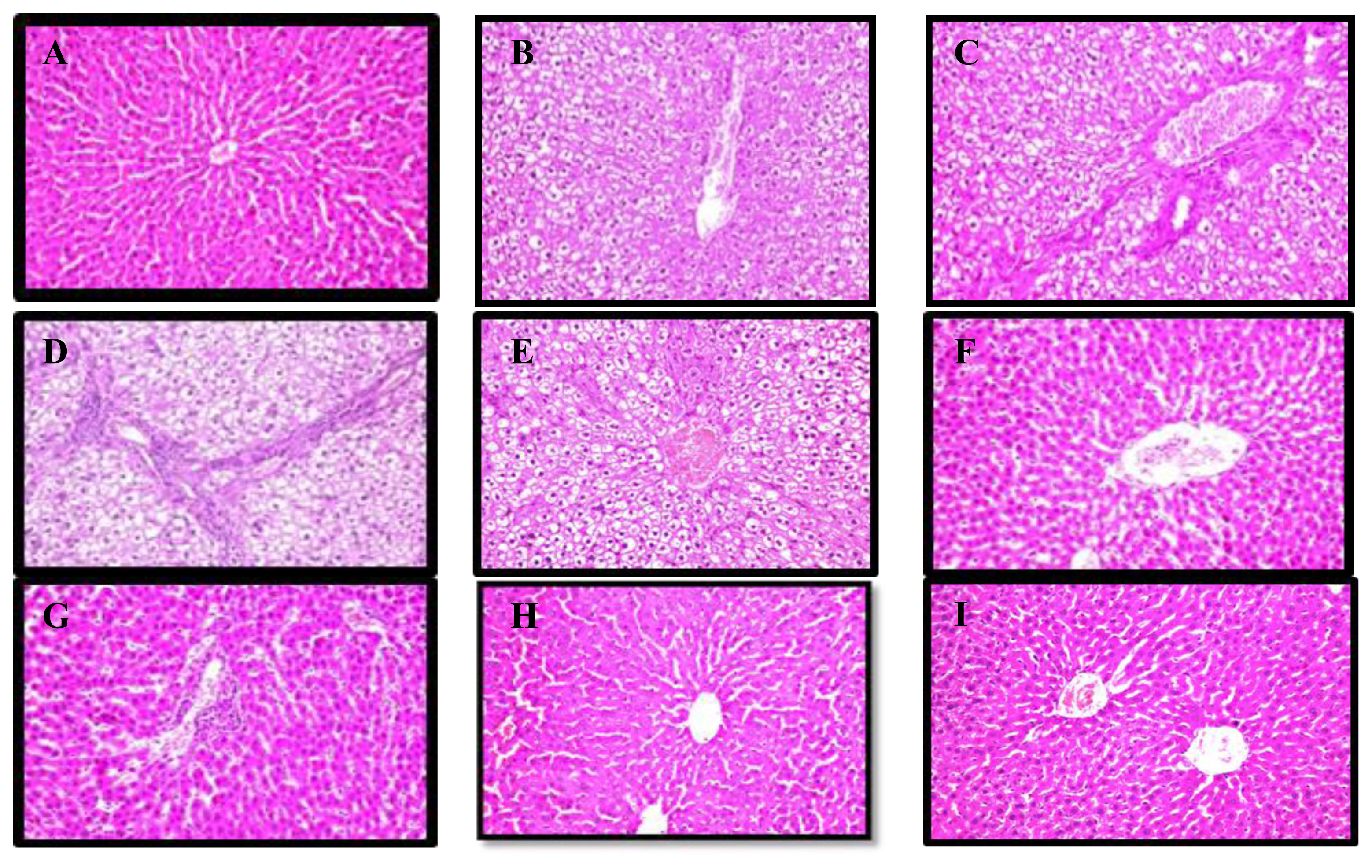
(A) Liver of control showing normal histological structure; (B and C) liver of IMI at 45 mg/kg (high dose) showing centrilobular necrosis and fibrosis with congestion; (D and E) liver of IMI at 22.5 mg/kg (low dose) showing necrosis with inflammatory in the cell; (F and G) Liver of IMI at 45 mg/kg plus algal polysaccharide (high dose+ algae) showing dilated portal vein with few inflammatory cells; (H) Liver of IMI at 22.5 mg/kg plus algal polysaccharide (low dose+algae) showing no histopathological alteration; (I) Live rat of algal polysaccharide showing the normal histological structure.
Conclusions
IMI exerts its toxic effects on liver cells by increasing oxidative stress and genotoxicity, whereas marine algae could alleviate redox cycle imbalances caused by the accumulation of free radicals in liver tissues. Natural polysaccharides from Rhodophyta showed potent antioxidant activity. Further studies on mammals treated with low doses of IMI or different doses of polysaccharides long term are necessary to confirm the findings of this work.
Acknowledgement
This project did not receive any specific grant from funding agencies.
Notes
We declare have no conflicts of interest.
CRediT author statement
HA: Conceptualization, Methodology, Software, Writing-Reviewing and Editing; AA: Data Curation; MF: Writing-Original draft preparation; NM: Writing-Original draft preparation; JG: Supervision; SM: Data Curation; EI: Methodology, Writing-Reviewing and Editing.
Abbreviations
IMI
Imidacloprid
TCA
phosphomolybdic acid
GSH
glutathione reduced
CAT
catalase
DPPH
2.2.diphenylpicrylhydrazyl
GPx
glutathione peroxidase
MDA
malondialdehyde
MN
micronucleus
FCS
Fetal calf serum
LPO
lipid peroxidation
TBA
thiobarbituric acid
MN
Micronucleus frequency
GR
glutathione reductase
GST
Glutathione S-transferases
AAPH
2,2′-azobis (2-amidinopropane) dihydrochloride
PUFA
polyunsaturated fatty acids
TBARS
Thiobarbituric acid reactive substances
ROS
Reactive oxygen species
HPA
hypothalamic-pituitary-adrenal
Nrf2
Nuclear factor erythroid 2-related factor 2
ERK
extracellular signal-regulated kinase
GSK-3β
Glycogen synthase kinase-3β
γ-GCS
Gamma-glutamylcysteine synthetase-glutathione
Caspase-9
cysteine-aspartic acid protease
Bax
Apoptosis regulator
JNK
c-Jun N-terminal kinases