Evaluation of genotoxic and oxidative stress potential of Ajakanga Landfill Leachate in rats
Article information
Abstract
Genotoxicity and oxidative stress potential of Ajakanga Landfill Leachate (ALL) were investigated in this study. Forty-two male albino rats of the Wistar strain (100 g and 150 g) were divided equally into six groups. Group A (control) animals were given distilled water as drinking water for forty-five days; while groups B-F animals were exposed to 12.5%, 25%, 50%, 75% and 100% leachate respectively via drinking water for forty-five days. The effect of the leachate was assessed on markers of oxidative stress in the liver, kidney and testes of rats; markers of liver function (Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) were determined in the serum and the genotoxic effect of the leachate was investigated using micronucleus assay. Physicochemical and heavy metal analysis were also carried out on the leachate sample. Exposure to ALL resulted in increase in the activities of ALT and AST. A significant increase in malondialdehyde level as well as alterations in antioxidant status was observed in the liver, kidney and testes of the rats compared with control. There was also significant increase in micronuclei formation in the bone marrow of rats exposed to the leachate. Physicochemical and heavy metal analysis of the leachate revealed the presence of some heavy metals at high concentrations as well as other toxic constituents and the total number of active bacteria in the leachate sample were also high. In conclusion, ALL induced oxidative stress and genotoxicity in rats. This suggests that the leachate may be toxic to humans if exposure occurs.
Introduction
The unsanitary disposal of municipal solid waste is of public and environmental health concern. Improper management of solid wastes is one of the environmental problems in developing countries. In many developing countries wastes are scattered around cities or dumped in dump sites [1]. Some of these dumpsites or landfills are located close to residential areas which is of great health concern as exposure to contaminants from landfills have been associated with several adverse effects including congenital abnormalities and neonatal deaths [2,3]. Solid wastes in dump sites or landfills undergo physical, chemical and microbial processes which results in the formation of leachate. Leachate formation is one of the many environmental hazards associated with landfilling because it contaminates the environment, particularly groundwater and surface water resources [4,5]. To protect water resources in the vicinity of landfills, it is necessary to maintain a low level of leachate water in the waste deposit [6]. The composition of leachates varies throughout the lifespan of the landfill, because of different chemical reactions that occur in the landfill and waste solubilization [7]. Heavy metals are one of the components of landfill leachate; in fact, leachate is classified as one of the main sources of heavy metal discharge into the environment [8]. Leachate discharged from landfills can contaminate ground water sources which is of great health concern, because most people drink untreated well-water and also use such for other domestic purposes [9].
To avoid water contamination and other adverse effects of leachates, in developed countries, landfills are designed with clay or plastic liners and leachate management setups to prevent the movement of leachate into the environment [10]. However, in most developing countries, most landfills do not meet the standards of those in developed countries [11].
Ibadan, the capital of Oyo State and the largest city in the south-western part of Nigeria generates about 1,618,293 kg of solid wastes daily [12]. With an estimated population of over 3 million, Ibadan is ranked third most populous Nigerian city [13].
Ajakanga Landfill has been in operation since 1996 till date. It is located along Odo Ona Elewe close to River Ona in Oluyole local government in Ibadan, Oyo State, Nigeria [14]. Due to rapid urbanization, residential areas with wells and bore holes as the major source of water are built close to the landfill. Untreated wastes are dumped daily in Ajakanga landfill, which makes ground water sources in the area prone to contamination, posing environmental risks and endangering the lives of people around the landfill. Hence there is need for the assessment of toxicity of leachate from Ajakanga Landfill.
Heavy metals and other components of leachates could be genotoxic. Exposure to genotoxic compounds can result in genomic instabilities and/or epigenetic alterations, which can cause several diseases including cancer [15]. Genotoxic compounds are sources of reactive oxygen species (ROS) which can generate free radicals in exposed tissues [16]. These free radicals can induce lipid peroxidation which can lead to different types of toxicity and cell death. An imbalance between the production and manifestation of reactive oxygen species and antioxidant system’s ability to neutralize the reactive intermediates or to repair the damage they have done results in oxidative stress [17]. This study investigated for the first time the genotoxicity and oxidative stress potential of Ajakanga Landfill leachate in male albino rats. The genotoxic effect of the leachate was assessed using micronucleus assay while oxidative stress indices were evaluated in the liver, kidney and testes of rats.
Materials and Methods
Study area and leachate sampling
The study area is Ajakanga Landfill site in Oluyole local government, southwestern part of Ibadan in Oyo State, Nigeria. Ajakanga Landfill lies between latitude 7°18′41.32″ North and Longitude 3°50′29.34″ East. It was opened in 1996 and is still in operation till date [12]. Raw leachate samples were collected from leachate wells on the site and thoroughly mixed. The leachate samples were filtered to remove debris and stored until use. The sample was designated Ajakanga Landfill Leachate (ALL).
Animal exposure and experimental design
Forty-two male albino rats of the Wistar strain weighing between 100 g and 150 g were obtained from a standard breeder (Emma Farms, Academy, Ibadan). The rats were divided equally into six groups. Group A (control) animals were given distilled water as drinking water; while groups B-F animals were exposed to 12.5%, 25%, 50%, 75% and 100% leachate respectively via drinking water for forty-five days to determine the sub chronic effect of the leachate. Generally, the purpose of the sub chronic toxicity test is to obtain information on possible toxic effects after repeated exposure to a test compound for a certain period of time, and information of the dose that does not cause toxic effects (No Observed Adverse Effect Level (NOAEL)) and to study the cumulative effects and reversibility of the toxic effects of the test compound. Several studies have evaluated the toxicity of various compounds using sub chronic toxicity test [18–20].
The rats were fed with commercially available standard pelleted feed and water ad libitum throughout the experimental period. Twenty-four hours after the last treatment, the rats were sacrificed by cervical dislocation and blood samples were collected from the retro orbital sinus of the rats into centrifuge tubes. Livers, kidneys and testes were immediately removed, washed in ice cold 1.15% potassium chloride solution, blotted and weighed.
Physicochemical parameters and heavy metal analysis of the leachate
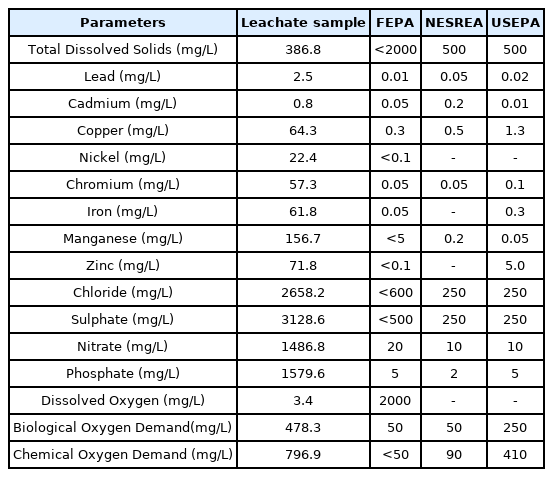
The leachate was analyzed for some physico-chemical parameters in accordance with standard methods (Federal Environmental Protection Agency, FEPA [21]; United States environmental protection agency, USEPA [22]. Some of the parameters analyzed include chemical oxygen demand (COD), biochemical oxygen demand (BOD), dissolved oxygen (DO), total alkalinity, total acidity and total hardness. The concentrations of metals such as copper, lead, iron, cadmium, chromium, nickel, manganese, mercury, arsenite and cobalt were also analyzed using atomic absorption spectrophotometer according to the American Public Health Association Standard methods for the examination of water and wastewater [23] and USEPA method [22].
Biochemical assays
The organs were homogenized in 4 volumes of homogenizing buffer (50 mM Tris-HCl mixed with 1.15% potassium chloride solution (pH adjusted to 7.4). The resulting homogenate was centrifuged at 12,500 g for 10 minutes at 4 °C to obtain the post mitochondrial supernatant fraction used for biochemical assays. Lipid peroxidation was measured in the organs as malondialdehyde (MDA) according to the method described by [24]. Reduced glutathione (GSH) concentration was determined in the organs according to the method of modified by [25,26]. Superoxide dismutase (SOD) activity was evaluated in the organs by the method of modified by [27,28]. Glutathione-S-transferase (GST) activity was determined in the organs according to the method described by [29]. Catalase activity was estimated in the organs according to the method of modified by [30,31].
In Vivo micronucleus assay technique
The frequency of micronucleated polychromatic erythrocytes (MnPCE) was estimated based on the method described by [32].
Statistical analysis
Results were expressed as mean±standard deviation. Student’s test was used to determine differences between groups and p-values<0.05 were considered statistically significant.
Results and Discussion
Concentration of heavy metals and other physicochemical parameters of ALL were not in compliance with federal environmental protection agency (FEPA), national environmental standards and regulation enforcement agency (NESREA) and United state environmental protection agency (USEPA) standards for industrial effluents and water quality. The total number of active bacteria in the leachate sample was also high, Tables 1a and 1b [22,33,34]. This result corroborates the results of [35] who earlier reported that concentrations of heavy metals in leachate and water samples from a stream around Ajakanga dumpsite were higher than WHO and FEPA standards. [15] also reported that the concentrations of some metals and physicochemical parameters of ground and surface water sources around the dump site were above permissible limits by NESREA and world health organization (WHO). Elevation in the physicochemical characteristics of ALL above normal values can cause severe degradation of groundwater quality and preclude its use for domestic water supply purposes. The high concentration of heavy metals in ALL is of great health concern as heavy metals have the potential to induce mutations and cancer in living cells.
There was concentration-dependent, significant (p<0.05) induction of micronucleated polychromatic erythrocytes (MNPCE) in rats exposed to the leachate Table 2. Micronuclei are formed apart from the main nucleus in cells as a result of acentric fragments or lagging chromosomes that do not incorporate into either of the daughter nuclei during cell division [36]. The observed increase in MNPCE in the bone marrow of the rats further reflects the toxic effect of the leachate and shows that some of the constituents of the leachate are cytotoxic, mostly the toxic metals as revealed from the results of physicochemical analysis of the leachate. Metals interact with DNA and nuclear proteins, which causes DNA damage, leading to cell cycle modulation, apoptosis or carcinogenesis [37]. This result is similar to the findings of [38] in mice exposed to leachates from Amilegbe dump site in Ilorin, Nigeria and also [39] who observed increase in MNPCE in the bone marrow cells of rats exposed to effluent from a pharmaceutical company.
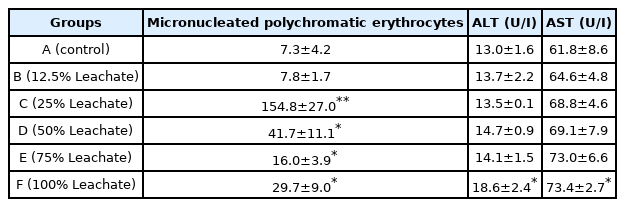
Micronuclei formation in bone marrow and serum activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
There was also increase in the activity of both ALT (alanine aminotransferase) and AST (aspartate aminotransferase), which are liver enzymes and markers of liver function in the serum of rats exposed to ALL when compared with the control Table 2. The activity of ALT and AST is increased in the serum or plasma during hepatocellular damage induced by drugs or diseases [40]. Elevated levels of ALT and AST in rats exposed to ALL shows that the leachate caused hepatic damage due to the induction of necrosis by toxic constituents of the leachate. This result corroborates previous reports of Bakare et al. (2012) [41] where there was increase in serum activities of ALT and AST in rats exposed to Olusosun and Aba-Eku municipal landfill leachates and Domínguez et al. (2019) [42] who also observed increase in serum activities of ALT and AST in mice exposed to electronic waste leachate.
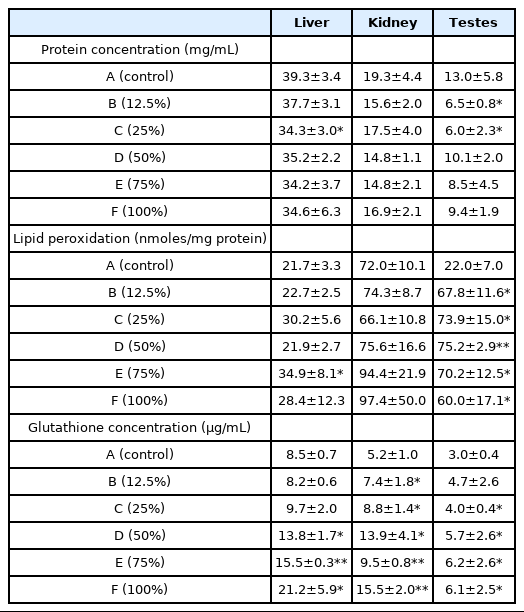
Li et al. [43] and Bakare et al. [44] reported that the possible mechanism of municipal landfill and sludge leachates induced genotoxicity and toxicity in mice was through oxidative damage. This is in line with the results of this study as exposure to ALL also led to induction of lipid peroxidation in the organs of the rats exposed to the leachate. Malondialdehyde (MDA) is an end product of lipid peroxidation which is a very complex process which involves different mechanisms and considered a biomarker of oxidative stress and cellular damage [45]. Malondialdehyde was significantly (p<0.05, p<0.001) elevated in the testes of rats after sub chronic exposure to ALL. There was also increase in MDA concentration in the liver and kidney of the rats although non-significant except in the liver of rats exposed to 75% concentration of the leachate (Table 3). The significant increase in MDA concentration in the testes of the rats suggests that the testes is a target of ALL induced toxicity, it is possible that some of the constituents of the leachate are testicular toxicants. This observation is similar to the findings of [46] who observed testicular and epididymal degeneration with significant decrease in sperm quality and quantity in rats exposed to Olusosun landfill leachate and [47] who also reported reproductive toxicities in male mice exposed to Olusosun landfill leachate. The observed increase in MDA concentration in the organs of the rats is consistent with the results of [48] in rats exposed to Eliozu land fill leachate in Port Harcourt.
The tripeptide, γ-l-glutamyl-l-cysteinyl-glycine known as glutathione (GSH), is an important low molecular weight antioxidant synthesized in cells. It is synthesized by the sequential addition of cysteine to glutamate followed by the addition of glycine [49]. Glutathione plays a crucial role in protecting cells from oxidative damage and during oxidative stress, there are changes in the concentrations of GSH. The increase in GSH concentration observed in the organs of the rats (Table 3) may be as a result of an induction mechanism for the neutralization of reactive oxygen species. High concentrations of GSH are important in attenuating oxidative damage to cells [20].
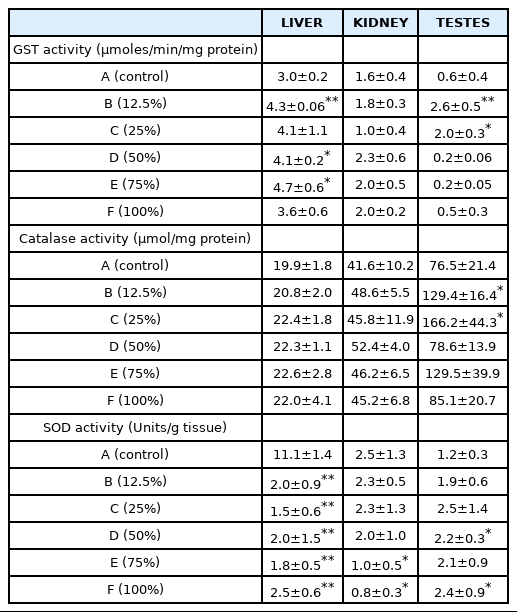
Glutathione transferases are multifunctional phase II detoxification enzymes involved in many catalytic and non-catalytic processes [50]. They play a central role in the detoxification of electrophilic chemicals and removal of potentially harmful hydrophobic compounds from blood. The activity of GST increased significantly (p<0.05; 0.001) in the liver, kidney and testes of rats after exposure to ALL indicating activation of detoxification mechanism (Table 4). Induction of GST activity is stimulated by large variety of pollutants including metals. Elevation in the activity of GST in the organs of the rats suggests an adaptive and protective role of this biomolecule against oxidative stress induced by ALL. This result is consistent with the result of our previous work in which there was increase in GST activity in the liver and kidney of rats exposed to Olushosun landfill leachate [20]. However, the explanation for the observed decrease in GST activity in the testes at 50%, 75% and 100% concentration of the leachate could be that the toxic constituents in the leachate overwhelmed the activity of the enzyme in the testes at high concentrations. This corroborates the earlier suggestion that some of the constituents of the leachate might be testicular toxicants from the observed significant increase in MDA concentration in the testes of the rats compared with the liver and the kidney.
Superoxide radicals formed through one electron reduction of molecular oxygen are rapidly converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD) [51]. However, superoxide radicals and hydrogen peroxide (H2O2) can cause the oxidation of cysteine which can eventually lead to decreased SOD activity [52]. This explains the decrease in the activities of SOD observed in the liver and kidney of rats exposed to the leachate, which may be as a result of high concentrations of heavy metals and other toxicants in the leachate; free radicals generated by these toxicants overwhelmed the activity of the enzyme which probably also resulted in an increase in catalase activity in these organs (Table 4). The product of SOD activity which is H2O2, a potent oxidant at high cellular concentration, probably led to the induction of catalase activity in the liver, kidney and testes of the rats. This is to counter the effect against increased oxidative stress. Our result is consistent with earlier reports of [42,48,53].
Conclusions
Increase in serum markers of hepatic damage, number of micronucleated polychromatic erythrocytes and malondialdehyde; coupled with alterations in antioxidant status in rats exposed to Ajakanga Landfill Leachate (ALL) show that the leachate induced genotoxicity and oxidative stress in the experimental animals suggesting the participation of free radicals/reactive oxygen species in the mechanism of ALL-induced toxicity. This is of great concern as people living around Ajakanga landfill site as well as their offsprings may be at great risk of several diseases including cancer. Daily exposure to leachates and its contaminants could possibly be responsible for the increase in the incidence of genetic, organ and blood related diseases. Therefore, government should consider re-designing of landfills with clay or plastic liners to prevent leachate from percolating into water bodies. Federal environmental protection agency should also implement better waste management practices that are environmentally friendly and siting of dumpsites close to residential areas or building houses close to established land fill sites should be discouraged.
Notes
CRediT author statement
OAA: Conceptualization, Visualization, Methodology, Supervision, Data Curation, Writing-Original draft Preparation; OON: Supervision, Writing-Reviewing& Editing; SCN: Investigation, Data Curation