The use of green mussel as bioindicator of heavy metal pollution in Indonesia: a review
Article information
Abstract
Asian green mussel is a marine animal that is used as food by most Indonesians. The mussels are widely cultivated in tropical countries such as Indonesia, Malaysia, Thailand, and other Asian countries. The mussel, known as perna viridis, is marine biota that is a filter feeder in the waters. Therefore, the quality of its meat is greatly influenced by the quality of the sea in its habitat. It is a food that is quite popular with the community but can endanger public health due to the accumulation of heavy metals. This study used a literature review by collecting data related to heavy metal concentrations in green mussel tissue in Indonesia. The results showed that the mussels from several sampling locations still exceeded the maximum acceptable limits of lead (Pb), mercury (Hg), and cadmium (Cd) concentration according to the standards of the food and drug administration of the Republic of Indonesia. Consumption of green mussels can increase health risks if you frequently consume them from cultivating or catching locations that have been contaminated with heavy metals.
Introduction
Asian green mussel is a marine animal that is used as food by most Indonesians. Cultivation of green mussels is found in various coastal areas of Indonesia. The high public interest in consuming the mussel makes it a salable commodity in the market. According to data from the central statistics agency in a report from the ministry of trade of the Republic of Indonesia, shellfish is one of the most commonly purchased types of food for consumption by people [1]. The culture of the Indonesian people who like seafood is also a factor in the high consumption of green mussels in this archipelago [2,3]. Green mussels are widely cultivated in tropical countries such as Indonesia, Malaysia, Thailand, and other Asian countries [4–7]. The cultivation is not influenced by the season so that it can be productive all year round [8]. The mussels can live in a variety of aquatic environmental conditions so that they are easier to cultivate. However, green mussels can accumulate water pollutants so that they can present health risks if consumed [9].
Asian green mussel, known as Perna viridis, is marine biota that is a filter feeder in the waters. Therefore, the quality of its meat is greatly influenced by the quality of the sea in its habitat. The green mussel tissue can accumulate various pollutants from the sea. The disposal of industrial waste in the sea can affect the increasing accumulation of heavy metals in the tissue. The ability to accumulate pollutants in water so is often used as a bioindicator of seawater quality. The accumulation of heavy metals in the green mussel tissue indicates heavy metal pollution in the sea [10]. Cultivation of seashells must ensure that the concentration of heavy metals in the waters has met the standards. For example, green mussels found in Jakarta Bay’s cultivation location show lead (Pb) concentrations of 0.202–0.289 mg/kg [11]. The concentration is still categorized as safe. If Pb exposure occurs continuously, it can increase the accumulation of Pb in green mussels.
The accumulation of heavy metals in green mussels is found in several marine areas in Southeast Asia. A total of 2.28 μg g−1 Pb and 8.96 μg g−1 copper (Cu) were found in green mussels in Johor, Malaysia [12]. Heavy metals have also been detected in green mussels off the coast of Singapore [13]. Heavy metals in green mussel tissue are also found in the West Pacific region [14]. The accumulation of heavy metals in the tissue can endanger human health if consumed for a long time. Moreover, heavy metals are non-essential to the human body, so they can be carcinogenic if they enter the body [10,15]. Industrial activities in coastal areas can endanger marine life. That can happen if the industry dumps waste that does not meet quality standards into the sea. The location of green mussel cultivation around the industry can increase the concentration of heavy metals in the body of green mussels. Several areas of cultivation and capture of mussels in Indonesia are located around industries and human activities that can produce pollutants. This is a danger signal for green mussel consumers because of the accumulation of heavy metals.
Asian green mussel is a food that is quite popular with the community but can endanger public health due to the accumulation of heavy metals. Therefore, we examine the concentration of heavy metals in green mussel tissue in the coastal areas, particularly the island of Java, Indonesia. This study aims to provide an overview of the ability of green mussels as a seawater bioindicator and the health risks associated with consuming green mussels that have been contaminated with heavy metals.
Methods
Data were collected through PubMed and Google Scholar with the keywords green mussel, sea pollution, and Indonesia. A total of 4 articles were obtained from PubMed and 263 articles from Google Scholar. A total of 7 full-text articles assessed for eligibility. The article was investigated regarding the concentration of Pb, Hg, and Cd in the green mussel tissue. Most of the sampling locations for green mussels are on the north coast of Java Island.
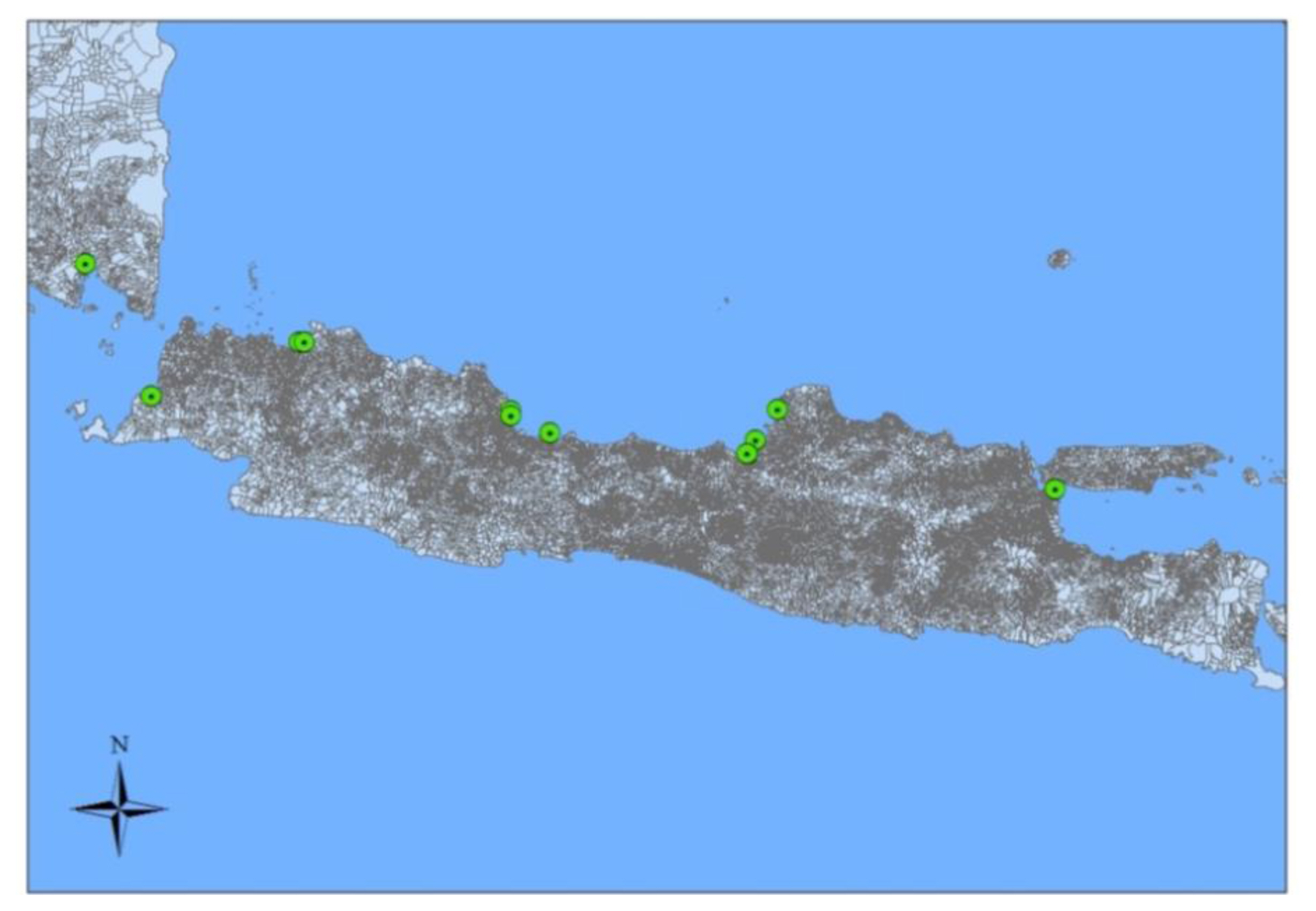
Based on the studies that have been carried out in the article, there are 12 sampling locations for green mussels. The sampling locations consisted of Bandar Lampung, Banten, Jakarta, Cirebon, Brebes, Semarang, Demak, Jepara, and Surabaya. There are locations whose samples were taken as many as two points, namely Jakarta, Cirebon, and Semarang. The location of the green mussel sample can be seen in Figure 1.
Green mussel as Pb, Hg, and Cd bioindicator
Based on the studies that have been carried out, the concentrations of Pb, Hg, and Cd in the green mussel tissue from the sampling location are as follows:
Based on the regulation of Indonesia food and drug administration number 5 of 2018, the maximum acceptable limit for Pb in green mussel tissue is 0.2 mg/kg [23]. Table 1 shows that the location for the sampling of green mussels that meet the requirements is Panimbangjaya Village. Pb concentration in the tissue’s Panimbangjaya Village was not detected. The highest Pb concentration in green mussel is found in Kali Baru and the industrial zone, Jakarta.
The concentration of Hg in green mussel tissue from several samples is still categorized as safe because it has not exceeded the maximum acceptable limit set by the food and drug administration of the Republic of Indonesia. The maximum acceptable limit for Hg in tissue is 0.5 mg/kg according to the standards of the food and drug administration of the Republic of Indonesia [23]. The Hg concentration that exceeds the standard in Kali Baru, Jakarta is 11.7 mg/kg.
The maximum acceptable limit on Cd in green mussel tissue is 0.1 mg/kg according to the regulations of the food and drug administration of the Republic of Indonesia [23]. Four sampling locations showed that the concentration of Cd in green mussel exceeded the maximum acceptable limit. The four sampling locations were Karangreja Village (Cirebon), Kali Baru (Jakarta). Bondet (Cirebon) and Jepara. Sources of pollution in the waters of Bondet, Cirebon, come from the industrial, market, and domestic waste [24]. Industrial waste is a source of pollution in Kali Baru (Jakarta) and Karangreja (Cirebon) [16]. The pollution in the waters of Jepara is influenced by industrial and domestic waste [20].
Green mussels are included in the bivalve group which is filter feeders in the water. All types of pollutants can be absorbed by shellfish so that they become bioindicators of water quality [25,26]. The pollutant that is absorbed by shellfish so that it accumulates in its body is called bioaccumulation. Various water pollutants can accumulate in the mussel. Even certain types of parasites can accumulate in shellfish so that they have the potential to be zoonotic if consumed by humans [27]. Heavy metals that are difficult to decompose in the waters cause these pollutants to accumulate in the body tissue [28]. The more anthropogenic pollutants that enter seawater, the greater their accumulation into the tissue. Human activities that produce waste in coastal areas can reduce the carrying capacity of the environment [29].
Scientists have recommended the use of shellfish as a bioindicator for decades. The nature of shellfish that remain in an ecosystem for a long time and have a low ability to metabolize pollutants in the waters makes it a bioindicator of water quality [30]. The use of shellfish as a bioindicator is better able to describe the quality of seawater in the past and present than using only sediment and water sample measurements. The level of accumulation of heavy metals in shellfish can be found in different waters [31]. Pollutant concentration factors and ecosystem conditions can influence it.
Which of the sediments, water, or shellfish are more capable of accumulating heavy metals in the sea? Several studies have shown that there is a difference between sediment, water, or shellfish in accumulating heavy metals. This is greatly influenced by the characteristics of pollutants and the condition of the aquatic ecosystem. Bivalvia can accumulate more heavy metals than sediment and water in Argentina’s coastal area [32]. Other studies in Egypt and Indonesia showed that the concentrations of Cd, Cu, and Zn were higher in the seashell tissue compared to sediments [10,33]. However, in contrast to the results of the study in Turkey, it showed that the concentrations of Cd, Cu, and Zn were higher in sediments than in other samples [34]. So it is difficult to conclude which of the three is better able to accumulate more metal. However, shellfish are more representative of long-term metal accumulation so they are used for seawater monitoring [35].
Some experts have explained that the examination of heavy metals in specific organs or tissues in shellfish is called a biomarker [36,37]. The organs that are used as biomarkers are the target tissue for accumulating heavy metals around it. The digestive glands can accumulate heavy metals so that they can be used as biomarkers [38]. Each aquatic biota has a different target tissue to be used as a biomarker. Green mussels are used as bioindicators to monitor heavy metal concentrations in seawater in several Southeast Asian countries especially Indonesia and Malaysia [38–42].
European countries also use shellfish as a bioindicator. cadmium concentration in shellfish in Gemlik Bay, Turkey, shows a value that exceeds the threshold required for consumption. The highest concentrations of heavy metals are found around industry and resorts around the bay of Gemlik [43]. Mining activities in coastal areas affect heavy metal contamination of shellfish. Mining activities in the Black Sea affect the Cu concentration in the tissue as required by the Turkish government [44]. Most of the shellfish found in coastal areas of Algeria is still safe from heavy metal accumulation [45]. Human activities in coastal areas have an influence on seawater quality and heavy metal contamination in these ecosystems.
Bioaccumulation of heavy metal and health risk
Green mussel is one of the most popular seafood in the world. However, there are health risks that can arise from consuming it. Bioaccumulation of heavy metal contamination in tissues can affect human health. Green mussels that have been exposed to heavy metals can present a carcinogenic and non-carcinogenic risk [46]. In assessing the amount of health risk caused by consuming green mussels that have been contaminated with heavy metals, Target Hazard Quotients (THQ) can be calculated. Several studies have shown that the concentration of heavy metals in tissues concerning THQ is still safe for human health. THQ measurement on the consumption of green mussel originating from Jakarta Bay shows the category safe for consumption [2]. THQ measurements in Algeria and Turkey also show the health risks posed by consuming shellfish are still safe for health [45,47].
Based on Figure 2, the entry of heavy metals into the food chain affects human health because humans are consumers of seafood. For example, green mussels exposed to heavy metals will accumulate in their bodies [48]. Heavy metals pollute the aquatic environment can enter green mussels tissues so that which is harmful to health if consumed by humans [22]. The green mussel intestine is the organ of the body that accumulates heavy metals [49]. Should not consume shellfish taken around the industry because it is susceptible to heavy metal contamination.
Prevention of exposure to heavy metals due to the consumption of shellfish must be a priority for governments in various countries. Efforts to prevent heavy metal contamination of shellfish must be started from the place where shells are raised. The location cultivation must be ensured that it is safe from exposure to heavy metals from anthropogenic activities around it [50]. This must be done to ensure public health, especially for those who enjoy seafood such as green mussels. The amount of shellfish consumption does not only occur in Southeast Asia but people in countries in the European continent also frequently consume it. The levels of heavy metal accumulation in Europe’s shellfish consumption have varying concentrations. However, the clam samples and THQ still showed a safe for health as shown in Italy [51]. Consumption of larger portions of shellfish for a longer period of time can increase the health risk due to exposure to heavy metals from the shellfish consumed.
The location of cultivation will determine the health risks of consuming green mussels. consuming shellfish at the sampling sites. Especially if you consume a larger portion of shellfish because it has been contaminated with Cd and Pb [52]. Heavy metal concentrations that accumulate in green mussel are influenced by the environmental conditions [53]. Therefore, ensuring environmental health at the cultivation location determines the level of heavy metal concentrations in shellfish. The amount of carcinogenic risk due to consuming shellfish is greatly influenced by daily intake. People are advised to consume shellfish no more than one serving a week to reduce daily intake [54]. It is recommended that the consumed green mussel come from cultivation locations or other coastal areas far from industrial areas and other anthropogenic activities.
Conclusions
Consumption of green mussels can increase the health risk if you often consume green mussels from breeding or harvesting locations that have been contaminated with heavy metals. Environmental quality monitoring efforts around the cultivation and catching of green mussels must be carried out routinely by the local government.
Notes
Conflict of interest
The authors declare that there is no conflict of interest in this article
CRediT author statement
IS: Conceptualization, Methodology; S: Data curation, Writing-Original draft preparation, VP: Writing - Review & Editing; H: Supervision, Validation