Acute toxicity assessment for TiO2 photocatalyst (GST) made from wastewater using TiCl4 in rat
Article information
Abstract
TiO2 was a photocatalyst that used to the most common product because of the high efficiency. TiO2 (P-25, commercial nanomaterial product) is the most typical photocatalyst product and TiO2 (GST) was a sludge recycling product. This study was reported to evaluate an acute toxicity of TiO2 (P-25 and GST) according to OECD test guideline 402 and 423 in Sprague-Dawley (SD) female rats via route of oral and dermal. There was investigated the lethal dose (LD50), and mortality, clinical signs, body weight changes and gross findings were continually monitored for 14 days following the single administration. After administration, TiO2 (P-25) was calculated that LD50 was considered to be a dose of over 2000 mg/kg body weight for both different route of exposure, and TiO2 (GST) was the same. Other items were no observed an adverse effect between P-25 and GST; no mortality and clinical signs, accidental body weight loss, no gross findings. On the basis of the above results, the toxicity of the GST was almost equal to that of the commercial product, P-25 and there was no toxicological evidence.
Introduction
Photocatalyst has been interested in worldwide because of its potential effect to use solar energy to solve environmental problems and provide a recyclable and sustainable energy [1]. One of many chemicals, titanium dioxide nanoparticles (TiO2 NPs) has been continuously studied for photocatalyst because it has a characterization such as high photocatalytic activity, excellent physical and chemical stability, low cost, non-corrosive, nontoxicity, and high availability [2–5].
Generally, TiO2 has been used within 200 ~ 400 nm particles to whiten or opacity many products (papers, toothpastes, sunscreens) and nanoparticles less than 100 nm were used in field of an automotive catalytic converter and UV protection agent, promoting either dispersion or resistance to photoactivity [6]. Recently, the commercialization of TiO2 has caused an increase of exposure to human and four main routes of exposure are known for oral / dermal exposures, pulmonary absorption and injection are known for one of the most common forms of route to human [7]. So far, there were performed steadily an acute toxicity study of TiO2 NPs of exposure on a various of route; inhalation, oral, skin (dermal, irritation, sensitization), injection, in vitro (human 3D model, etc.), etc. [8–12]. Although limited data are available on toxicological hazards of nanomaterials following oral exposure, in the oral route, the acute toxicity performed for TiO2 NPs in rodents, it showed that lethal dose (LD50) value was higher than 2150 mg/kg body weight or even 5000 mg/kg body weight [8]. The study for respiratory system of TiO2 was well-demonstrated, and the reports for other routes were not so much compared with inhalation toxicity [13,14]. Also, there were not much toxicity study for various routes in accordance with test guideline. In particular, no studies relevant for the assessment of acute dermal toxicity of nano-TiO2 are available [9].
The present study was performed to acute oral or dermal toxicity for new TiO2 catalyst, GST produced from the precipitated sludge using TiCl4 coagulants in the sewage treatment plants [16,17] in accordance with OECD test guideline and was compared with P-25 (AEROXIDE®, Evonik Industries, Germany), generally used in catalytic and photocatalytic industrial products.
Materials and Methods
Test facility
This study was conducted in compliance with the principles of Good Laboratory Practice (GLP) at KTR (Korea Testing & Research Institute), Hwasun based on the Korea Good Laboratory Practice (KGLP) and Organization for Economic Co-operation and Development (OECD) “Principles of Good Laboratory Practice, ENV/MC/CHEM (98)17 (as revised in 1997)”. Also, this study protocol was review and approved (IAC2019-2078, 2079, 2165, 2166) by the Institutional Animal Care and Use Committee (IACUC) of KTR Hwasun based on the Animal Protection Act. and the Laboratory Animal Act.
Acute oral toxicity study was conducted in accordance with the OECD guidelines for the testing of chemicals, section 4, TG 423 “acute oral toxicity-acute toxic class method” and acute dermal toxicity was conducted in accordance with the OECD guidelines for the testing of chemicals, section 4, TG 402 “acute dermal toxicity: fixed dose procedure” [18,19].
Experimental animals and housing
Sprague-Dawely (SD) rats were purchase from the Orient Bio Co., Ltd. in republic of Korea for acute oral (n=12, only female; 202.7 – 255.8 g) and for acute dermal (n=5, only female; 242.1 – 299.4 g), and were nulliparous and non-pregnant. The animals were examined for quarantine on the receipt and acclimatized for 7 days to the laboratory conditions. The animals were housed under condition for temperature (22 ± 3 °C), relative humidity (50 ± 20%), and 12-hour light/dark cycle. The animals were fed rodent diet 20 5053 [Labdiet, USA] and given the R/O water ad libitum. Healthy animals were selected and used for the study based on general health conditions.
Preparation of titanium dioxide (TiO2)
The titanium dioxide (TiO2), GST was manufactured from precipitated sludge recycling of the sewage treatment plant and P-25, commercial nanomaterial product (AEROXIDE®, Evonik Industries, Germany) was provided by Bentec Frontier Co., Ltd. The crystalline composition in X-ray diffraction (XRD) analysis was performed by the Research Institute for Catalysis in Chonnam National University as follows; GST –100 anatase, P-25 - 88% anatase/12% rutile.
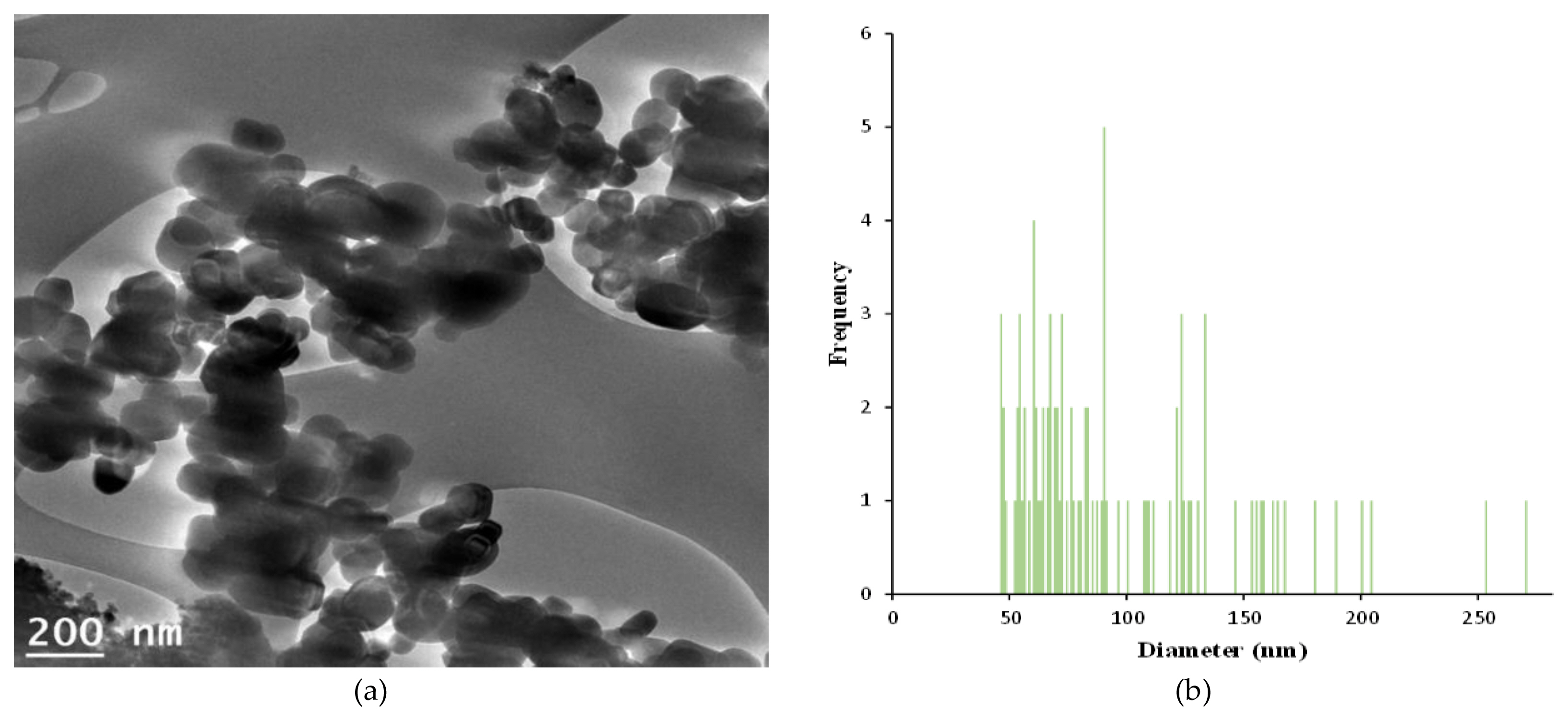
Also, the characterization of GST was determined using a Zeta potential [Particle size & Zeta potential analyzer (Zetasizer Nano ZSP, Malvern Instruments LTD., UK) in Korea TECH], FE-SEM (Field Emission Scanning Electrong Microscope, Tescan Corp., Czech) with EDS systems (Thermo scientific, USA) by center for advanced specialty chemicals (Ulsan) in Korea research institute of chemical technology (KRICT), TEM (Transmission electron microscopy) image [FE-EF-TEM (Field Emission Energy Filtered Transmission Electron Microscopy, JEOL, Japan) in Korea basic science institute (Jeonju department)] and size distribution [Image J software (https://imagej.nih.gov/ij/download.html)]. For the vehicle test, the test substances were suspended to distilled water (D.W., GST) and 0.5% (w/v) Tween 80 (P-25), respectively.
Toxicity procedure
Properties of GST
The characterization of GST showed in (Figure 1, 2 and 3), respectively. The zeta potential is an analytical technique for the determination of surface charge of nanoparticles in colloidal solution and gives a prediction of the colloidal stability. The values with > +25 mV or < −25 mV have high degree of colloidal stability and values more positive than +30 mV and more negative than −30 mV indicate good stability against coalescence [20, 21]. The result showed that it was a negative value (−35.4 mV), and GST was thought to be good stability and had enough property to be less agglomeration. Also, The value of particle size was 95.8±46.3 nm (mean±standard deviation) and 34.1% by number of the particles had a diameter > 100 nm. The nanomaterial was generally known as a small structure of 1 to 100 nanometers. Considering the size range of nanomaterial, the particle size for GST was thought that it was not a nanomaterial due to in various size.
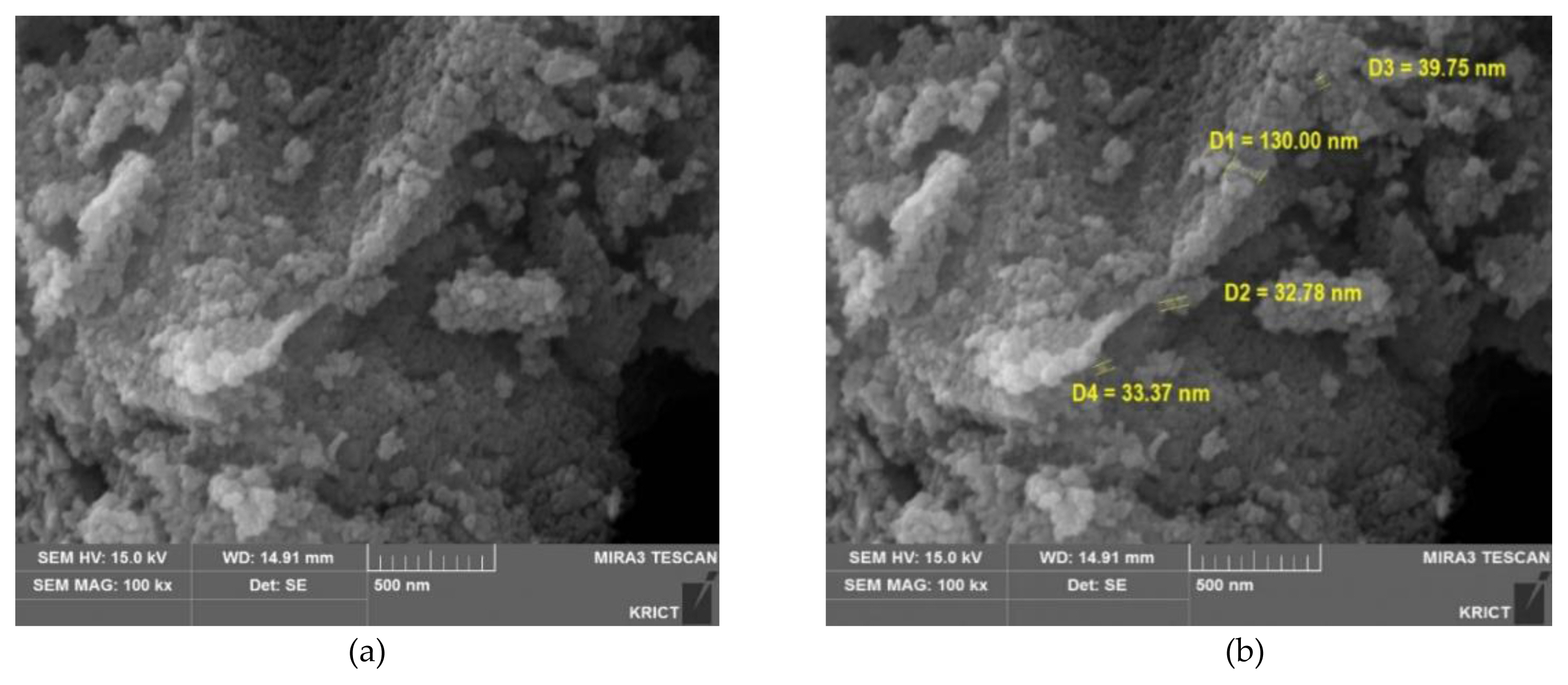
Image of titanium dioxide (TiO2) GST by scanning electron microscope (SEM): (a) Morphology of the TiO2; (b) Primary particle size of the TiO2.
Acute oral toxicity study
An acute dose of the test substance was sequentially administered by oral route at a dose of 300 mg/kg body weight (1st, 2nd step) and 2000 mg/kg body weight (3rd, 4th step) in accordance with test guideline [19] and three animals (9 – 10 weeks old) were used. The test substance was administered a single dose in a volume not to exceed 10 mL/kg body weight after over-night fasting (about 18 hours). The animals had been observed for 14 days after administration and mortality, clinical signs, body weight and necropsy findings were recorded. Clinical signs and mortality were observed once a day, and observed for 0.5, 1, 2, 3 and 4 hours on administration day and then once each day for 14 days. Body weight changes were measured at animal receipt day, animal allocation day, just before treatment and on day 1, 3, 7 and 14 after the administration. At the end of observation period, external observations were conducted and sacrificed by bloodletting under anesthesia using isoflurane (Ifran Liquid for Inhalation, Hana Pharm. Co., Ltd.). Then, all organs including major organs were examined for gross lesions.
Acute dermal toxicity study
An acute dose of the test substance was sequentially administered by dermal route at a dose of 200, 1000, 2000 mg/kg body weight (Range-finding study, 3 steps in total) and 2000 mg/kg body weight (Main study, 1 step in total) in accordance with test guideline [18]. The 5 animals were used for range-finding study (8 – 9 weeks old, three) and main study (9 weeks old, two). On the day prior to the application, the back of each animal was clipped free of hairs and was approximately 10% of the total body surface area. The test substances were applied with a single dose in a volume not to exceed 5 mL/kg body weight and were held in contact with the skin with a porous gauze dressing (Coban, 3M) and non-irritating tape (Tegaderm™ Film, 3M) during a 24-hour exposure period. The wrappings and the adhesive bands were removed at 24 hours after application and then the applied sites were washed out gently with water for injection. The animals were observed for 14 days after administration and mortality, clinical signs, body weight and necropsy findings were recorded. Clinical signs were carefully observed once a day for 14 days after administration. Only, administration day were observed at 0.5, 1, 2, 3, 4, 5 and 6 hours after administration. Body weights were measured at animal receipt day, animal allocation day, just before treatment and on day 7 and 14 after the administration. At the end of observation period, external and necropsy findings were performed as same as processing in oral toxicity.
GHS category
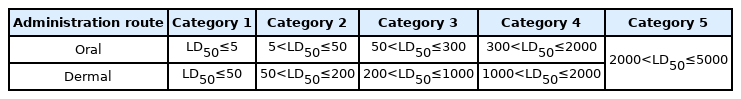
The substances were classified to criteria of globally harmonized system of classification and labelling of chemicals (GHS) category (Category 1 to 5 or unclassified) as follows in (Table 1). In the case of unclassified category, it was classified when no dead animals were observed in sequential two times (oral test) and were observed (dermal test) at 2000 mg/kg body weigh [22].
Results and Discussion
Acute oral toxicity
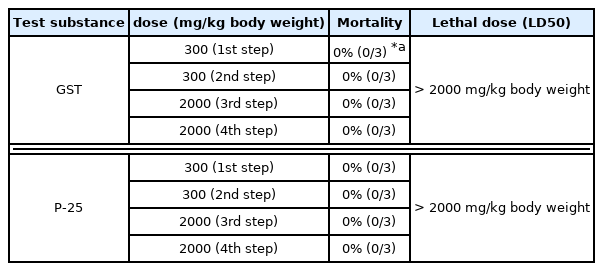
This study was executed to evaluate oral toxicity response and Lethal Dose (LD50) of the test substance, GST and P-25. When TiO2 NPs (P-25) and TiO2 (GST) were administered for acute oral administration to female SD rats, there were no mortalities and test substance-related signs during experimental period (Table 2). Body weight was decreased temporarily in some animals of each step of 300 mg/kg body weight (1st, 2nd step) and 2000 mg/kg body weight (4th step) after administration (Figure 4 and 5) considering to no abnormal clinical signs and degree of body weight loss (GST: 0.1 – 3.8%, P-25: 0.1 – 0.2%). Also, the necropsy for all animals showed no gross examinations for external and internal findings. These results indicated that TiO2 NPs (P-25) and TiO2 (GST) was considered to be no treatment-related effects and GHS category was classified as “GHS category 5 or unclassified”. From a risk assessment perspective, it is essential to point out that both size-dependent properties and biological effects that are of potential concern for human health, specifically toxicokinetic behavior and particle-cell interactions, are not closely related to specific size thresholds. In line with the EFSA scientific committee (2011a), the EFSA scientific committee reiterates that not all nanomaterials have new hazard properties compared with larger sized counterparts and that therefore a case-by-case assessment is necessary.
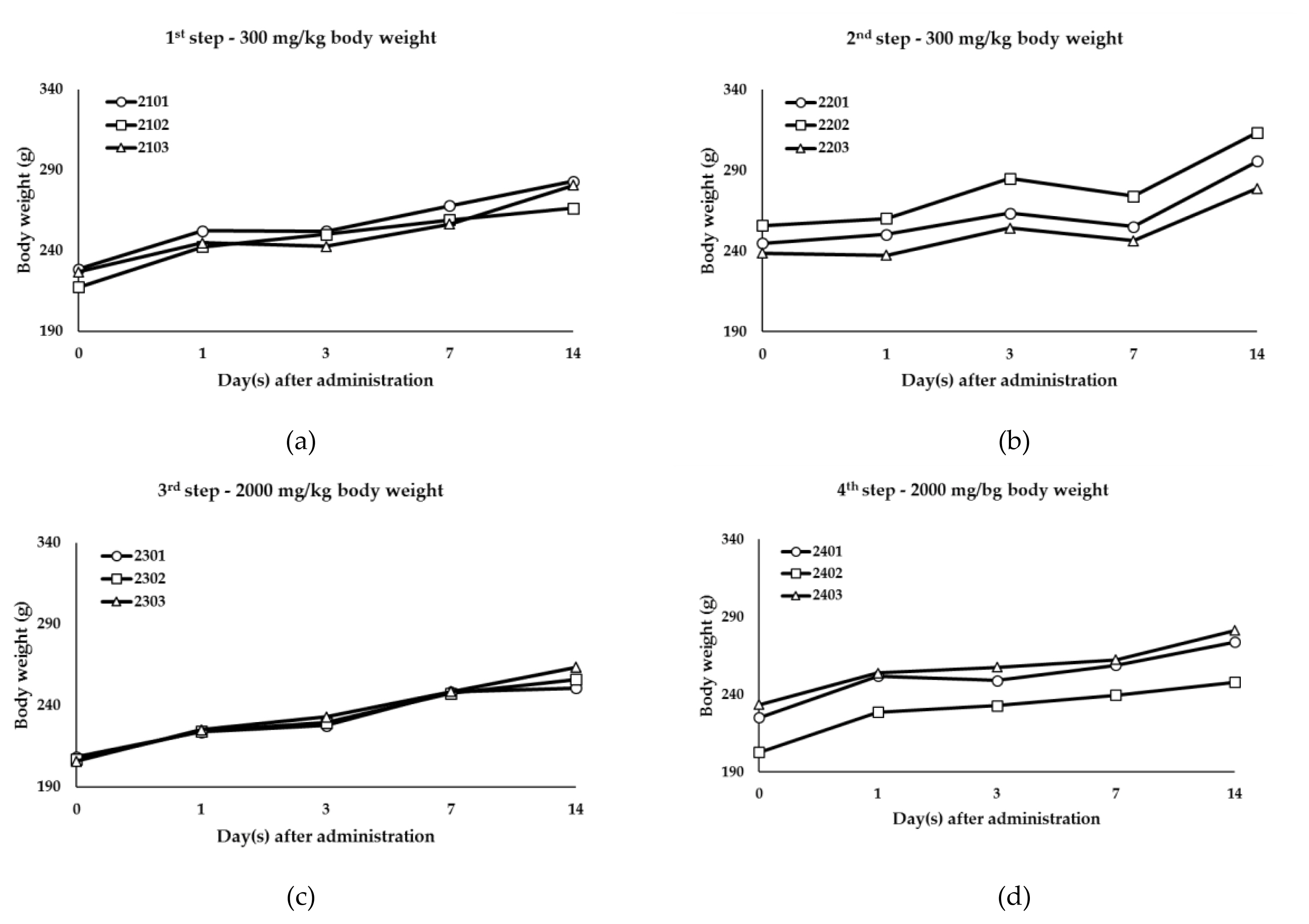
Body weight changes of titanium oxide (TiO2) GST: (a) Body weight of the 300 mg/kg body weight (1st step); (b) Body weight of the 300 mg/kg body weight (2nd step); (c) Body weight of the 2000 mg/kg body weight (3rd step); (d) Body weight of the 2000 mg/kg body weight (4th step).
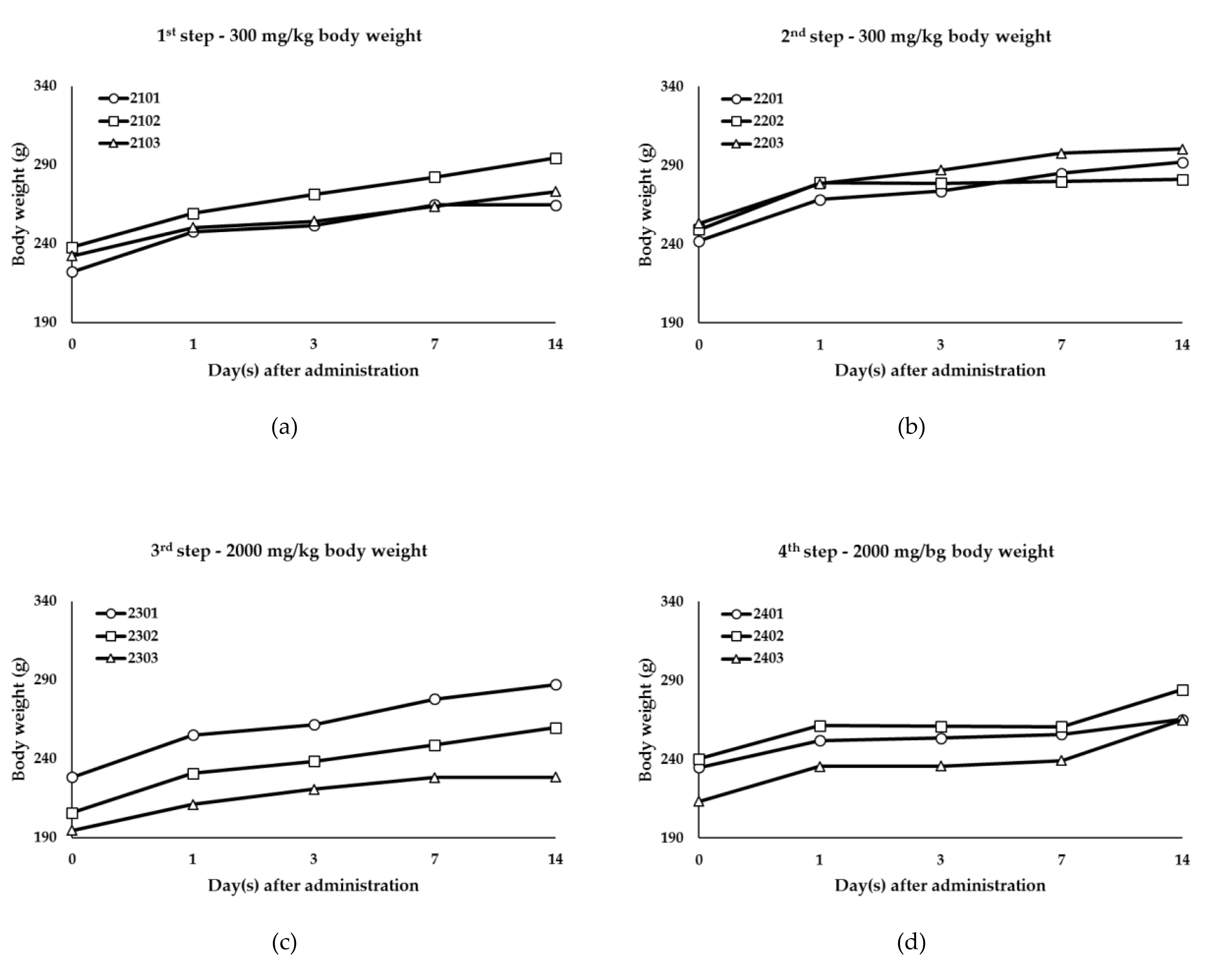
Body weight changes of titanium oxide (TiO2) P-25: (a) Body weight of the 300 mg/kg body weight (1st step); (b) Body weight of the 300 mg/kg body weight (2nd step); (c) Body weight of the 2000 mg/kg body weight (3rd step); (d) Body weight of the 2000 mg/kg body weight (4th step).
Wang et al. reported the treatment-related effect in acute toxicity (5000 mg/kg B.W.) as follows; increase liver weight, hepatic inflammatory response, histopathological alterations of the liver and kidneys, and increased levels of enzymatic biomarkers of cardiac lesions [14]. Considering this study, further study (repeated oral toxicity study, etc.) is thought to be needed to confirm whether presence or treatment-related effect for GST is observed or not.
Acute dermal toxicity
This study was performed to evaluate dermal toxicity response and Lethal Dose (LD50) of the test substance, TiO2 NPs (P-25) and TiO2 (GST). When TiO2 were administered for acute dermal administration to female SD rats, there were no mortalities and test substance-related clinical signs during experimental period on both test substances (Table 3). The body weight for GST showed normal increase body weight in all treatment animals during study period. (Figure 6) and temporarily decrease was observed on day 14 in Range-finding study of P-25 (200 mg/kg body weight) (Figure 7). However, it was no treatment-related effect due to the no abnormal clinical signs and degree of loss (P-25: 5.2%). Also, no gross findings for external and internal findings were observed in all treatment animals. Based on the result of acute dermal toxicity, NPs (P-25) and TiO2 (GST) was classified as “GHS category 5 or unclassified”.

Body weight changes of Titanium oxide (TiO2) GST: (a) Body weight of the Range-Finding study; (b) Body weight of the Main study.
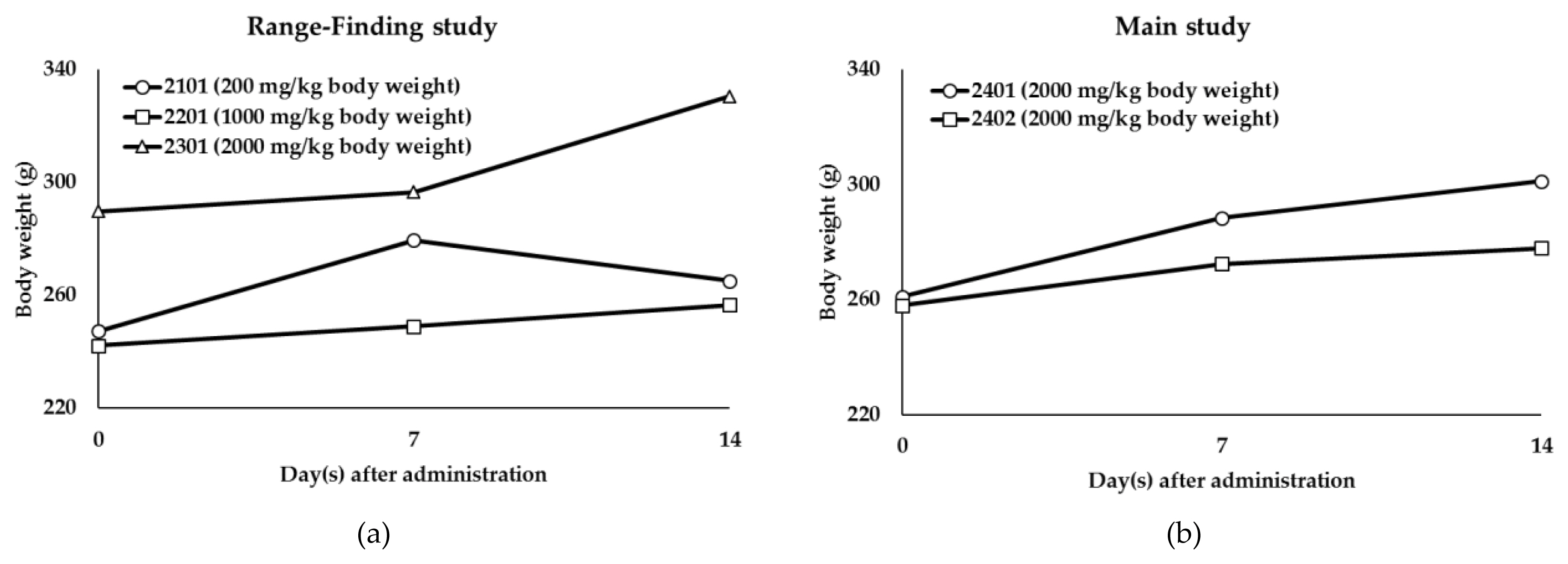
Body weight changes of titanium oxide (TiO2) P-25: (a) Body weight of the range-finding study; (b) Body weight of the main study.
Most of the reported studies are data on skin (dermal) absorption study of in vivo and in vitro and there is very little data about acute toxicity (dermal in particular) in accordance with test guideline on acute dermal toxicity. Considering reported studies, the present study is considered to be a good data of acute dermal toxicity test for TiO2.
Conclusions
In this study, there was performed to evaluate a potential hazard characterization and risk assessment for acute oral or dermal of TiO2, GST used as a photocatalyst. As the result of the acute toxicity, there were no toxicological evidence in mortality, treatment-related clinical signs, and necropsy findings for oral or dermal route. Therefore, it was considered that the lethal dose (LD50) was over 2000 mg/kg body weight and GHS category was “category 5 or unclassified”. Also, the acute safety of GST was thought to be no difference compared with P-25, commercial nanomaterial product. In the future, it seems that additional research on repeated dose toxicity study for estimate of no-observed-adverse-effect level (NOAEL) are needed, and also reproductive and development toxicity.
Acknowledgement
This work was supported by a grant (19SCIP-B145906-02) from the Korea Agency for Infrastructure Technology Advancement (KAIA) by Ministry of Land, Infrastructure and Transport of Korea government (MOLIT), Republic of Korea.
Notes
The authors declare that they have no conflict of interest.
CRediT author statement
JKS: Methodology, Data curation, Writing-Original draft preparation; MKP: Supervision, Writing-Reviewing and Editing; JMI: Visualization; HSS: Investigation; HJP: Resources; SSN: Project administration, Writing-Reviewing and Editing