Risk assessment via oral and dermal pathways from heavy metal polluted water of Kolleru lake - A Ramsar wetland in Andhra Pradesh, India
Article information
Abstract
The Kolleru Lake, India is a famous wetland of international significance. Analyses of certain potentially toxic heavy metal ions in water indicate that this freshwater lake is characterized by highly heterogeneous distribution of chromium (Cr; 4.5–80 μg/L), copper, iron (Cu, Fe; below detection limit), manganese (Mn; 1–313 μg/L) and zinc (Zn; below detection limit). Non-carcinogenic health risk assessment indices like hazard quotients (HQ) and hazard indices (HI) are estimated following the guidelines recommended by the US Environmental Protection Agency (USEPA). These indices are found to be within the acceptable limit (<1), indicating negligible potential health risk via ingestion and dermal routes. However, when the average values of these indices pertaining to the Kolleru lake are normalized with similar estimates from clean and uncontaminated global surface water, both high and low ratios are obtained. While Cr (12.5), Cu (2.3) and Mn (3.7) exhibit high ratios, those of Fe (0.09) and Zn (0.99) show respectively low and comparable values. The significance of such heterogeneous distribution of hazard indices and their ratios are discussed. Further, average carcinogenic risk levels of the adults and children due to Cr ingestion are estimated to be 0.00154 and 0.0022, respectively. Both values are higher than the permissible levels recommended by the USEPA. As a remediation measure, it is recommended that monitoring the levels of heavy metal in water and other items like fish in the lake or rice and vegetables grown in the area is needed to be carried out at regular intervals. This study therefore offers requisite perception to the local government and health officials to evolve their plan of action so that effective management and mitigation of water quality of the Kolleru lake can be administered.
Introduction
Over the past three decades, the environment has progressively deteriorated leading to air, water, and soil pollution. Rapid industrialization and diverse-natured anthropogenic activities are the major causes contributing to quality decline in most of the cases [1–4]. In addition, geogenic sources (naturally occurring) are also cited as contaminants in some instances [5,6]. It has been estimated that about one third of people in the world are deprived of safe drinking water [7]. Contaminated water and poor sanitation are linked to transmission of many diseases like cholera, dysentery, hepatitis A, typhoid, etc. [7]. Contaminants in drinking water are classified into four categories: (i) physical contaminants (sediment or organic material suspended in water), (ii) chemical contaminants that are either naturally occurring or anthropogenic (e. g., nitrogen, bleach, salts, pesticides, metals and heavy metals, toxins produced by bacteria, and human or animal drugs), (iii) biological contaminants (i.e., organisms in water such as bacteria, viruses, protozoan, parasites, etc), and (iv) radiological contaminants (elements made up of atoms having unstable nuclei that emit ionizing radiation).
Safe Drinking Water Committees under the aegis of the National Research Council in the US addressed the issue related to contamination of drinking water during 1977–1987. They evolved a systematic scientific and administrative scheme to assess health risks associated with exposure to toxic chemicals in drinking water [8–10]. Subsequently many studies were conducted globally to assess the quality of drinking water and health risks associated with ingestion of contaminated groundwater and surface water [3,4,6,11,12]. Health risk caused by heavy metals in a water source is an active area of research in recent years. This is because, depending on the heavy metal and its chemical form in water, prolonged exposure can affect target tissues such as brain, liver, bones, and kidneys in the human body resulting in serious health problems [13]. Therefore, health risk assessment is an essential part, where an evaluation is made to assess the possible health effects of groundwater and/or surface water bodies that are contaminated.
Among the surface water bodies, lakes provide livelihood to many people across the globe. In developing countries, more than 60 million people rely on lakes for their income [14]. However, pollution of lake ecosystem and its surroundings due to anthropogenic activities has become a major global issue in many parts of the world [15,16]. In India, the dependency on lakes for livelihood is also seen in different parts as evidenced from the explosion of agricultural and livestock farming, fishery industries and tourism around many lakes of the country [17–19]. All these Indian lakes are subjected to human-induced adverse impacts, which are undesirable.
The Kolleru lake is one of the largest natural freshwater lakes in India located in the southern state of Andhra Pradesh. In August 2002, the lake has been designated as a wetland of international importance [20] under the International Ramsar Convention. Wetlands are of considerable economic importance, as they nourish diverse biota and are natural habitat for a large variety of resident and migratory birds. Naturally, the Kolleru lake was also a “paradise of birds” and used to be a place of tourist attraction. However, during the past two decades the lake habitat has degraded significantly owing to various anthropogenic activities around its vicinity that include intensive agricultural, industrial and farm activities [21–25]. Despite being one of the largest freshwater lakes, it is irony that the area surrounding the Kolleru lake has acute shortage of drinking water [26]. Moreover, there is extreme poverty in the region that still persists [27]. It is therefore likely that majority of poor residents are forced to use lake water for drinking and other purposes.
As pointed out above, a number of studies were conducted to estimate the quality of Kolleru lake water and sediments, besides digital processing of IRS-1D LISS-III sensor data of the lake region [21–25]. However, no work following the guidelines recommended by the US Environmental Protection Agency (USEPA) on human health risk assessment has been attempted so far. In view of this, health hazard risk assessment is carried out in this study. Concentrations of six heavy metals in the Kolleru lake water that include cadmium (Cd), chromium (Cr), copper (Cu), iron (Fe), manganese (Mn), and Zinc (Zn) were analysed. Non-carcinogenic health risks associated with these heavy metals are estimated due to drinking and dermal absorption for general adults and children in the community. An attempt is also made to estimate cancerous health risk associated with ingestion of lake water contaminated with carcinogenic toxic metals. Significantly, the results of this new research may provide some insight to the local government and health professionals to evolve strategies to effectively manage water quality, and hence to revive the pristine glory of the Kolleru lake ecosystem.
Materials and Methods
Study area
In view of international significance of the area (Ramsar site), a brief account of the study area is presented first. Kolleru lake, located between the latitudes 16°32′ and 16°47′N and longitudes 81°05′ and 81°27′E, is the largest natural freshwater lake in the southern state of Andhra Pradesh. The lake lies between the deltas of two main rivers, Godavari and Krishna and it is approximately 35 km inland from the present shoreline (Figure 1). There are three main streams that drain into the lake. These streams are known as Budimeru, Thammileru and Ramileru. The lake finally drains itself to the Bay of Bengal through Upputeru river [24,25]. It is a shallow freshwater body with a natural spread of ~674 km2. However, its areal extent goes up to ~954 km2 during the rainy seasons when highest floods are recorded in the area. On the other hand, during dry seasons the areal extent comes down to as low as ~66 km2. It is significant to note that the lake also serves as a natural flood-balancing reservoir for the two major rivers– Godavari and Krishna. Fluvio–marine deposits (sand, silt and clay) characterize the lake surroundings from all sides. The nearest rock formations occur about 10 km away from the lake. The geological formations include khondalite, Gondwana, Deccan traps and tertiary sediments [28].
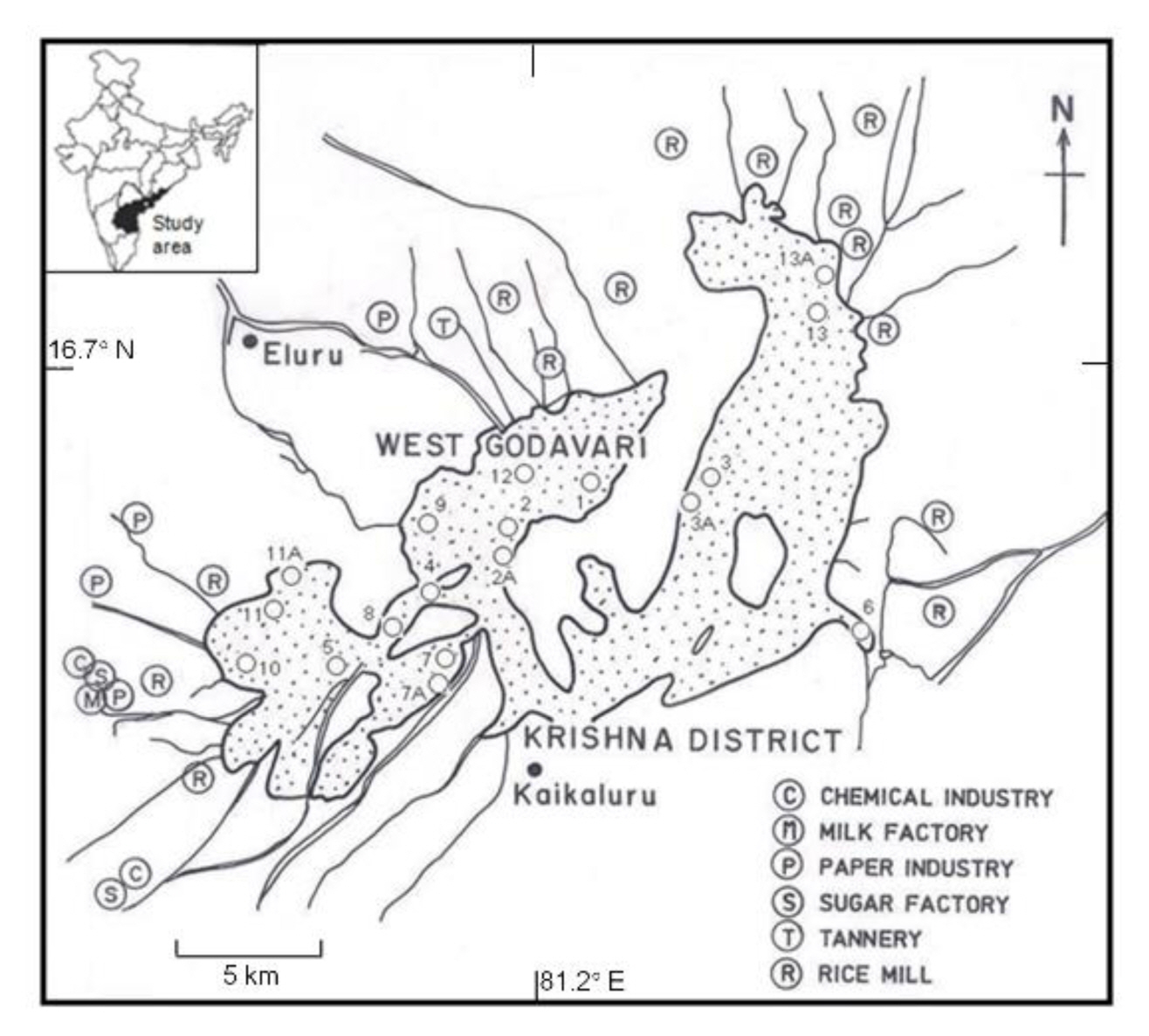
Map showing Kolleru Lake. Two prominent towns close to the lake, viz., Eluru and Kaikaluru together with various anthropogenic inputs surrounding the lake area [21] are shown. Eighteen sampling points for water are marked by open circles. The horizontal scale (5 km) displayed in the figure corresponds to 0.045º. The geographical location of the lake (study area) in the state of Andhra Pradesh, India is marked in the inset.
Sample collection, preparation, and analysis
Eighteen sampling points were selected to cover the lake area (Figure 1). Uniform sampling could not be carried out due to inaccessibility of some of the locations. The water samples were collected following standard procedure [29]. Polypropylene bottles (1 L) for sample collection were soaked in 5% HNO3 for 24 h followed by rinsing them a number of times with deionized water. The bottles were dried and water samples were collected in duplicate after filtration using Whatman No. 40 filter paper. The pH and total dissolved solids (TDS) were measured in the field. The pH electrode was calibrated with pH 4 and pH 9.2 buffer solutions. The samples were then acidified with 5 mL of HNO3 to prevent the adsorption of heavy metals onto the bottle walls and transported to the laboratory. Until use, preservation of these water samples was done following the recommendations of USEPA [29]. The heavy metal analyses were done in the laboratory within 2 to 3 weeks of the sample collection. The concentrations of cations such as Cd, Cr, Cu, Fe, Mn, and Zn were determined by ICP-OES (model Optima 4300 DV; PerkinElmer Life and Analytical Sciences, Shelton, CT, USA) using the standard procedure [30]. The instrument is equipped with a cross-flow nebulizer, Scott spray chamber, echelle grating, and segmented array charge-coupled device detector. The basic principle of operation of ICP-OES is that depending on the heavy metal contents in the sample, each element emits energy at specific wavelengths. However, based on the intensity of the emitted waves it is generally common to select a single wavelength for a given element. The intensity of energy emitted at the chosen wavelength is proportional to the concentration of the element of interest in the sample. It is thus possible to quantify the concentration of the element of interest in the sample relative to a reference standard using a calibration curve. During the course of analysis, a multi-elemental solution from Spex Certiprep, WP-15-500, (Spex, Metuchen, NJ, USA) was used for calibration. A process blank was also prepared, which was run along with samples and corrected for matrix effects.
Governing equations for health risk assessment
According to the USEPA, “a human health risk assessment is the process to estimate the nature and probability of adverse health effects in humans who may be exposed to chemicals in contaminated environmental media, now or in the future”[31]. Pollutants such as Cd, Cr, Cu, Fe, Mn, and Zn were selected in this study. The health risk assessment of each heavy metal contaminant in water is based on the estimation of the risk level. The methods delineated by the USEPA [32,33] have been used to estimate the average chronic daily intake (CDI) from direct ingestion and dermal absorption routes, respectively. The governing equations are as follows:
where CDIingestion/dermal is expressed in μg/kg/day; Cw is the measured concentration of chemicals in water (μg/L); IR is the ingestion rate (3.5 L per day for adults [34]; 1.32 L per day for children [35]); EF represents the exposure frequency (EF = 365 days per year); ED is the exposure duration (ED = 70 years for carcinogenic risk and 30 years for non-carcinogenic risk for adults; 6 years for non-carcinogenic risk for children [32, 33]); BW is the average body weight (57.5 kg for adults [36] and 15 kg for children [32,33]); AT refers to the average time representing the period over which exposure is averaged (AT = 25,550 days for carcinogenic risk and 10950 days for non-carcinogenic risk for adults; 2190 days for children [32,33]); SA is exposed skin area available for contact (18000 cm2 for adults; 6600 cm2 for children [33]); ET is exposure time (0.58 h/day for adults; 1 h/day for children [33]); CF is unit conversion factor (0.001 L/cm3 [33]); and Kp is dermal permeability coefficient (cm/h) of heavy metal in water [33].
The hazard quotient (HQ) is an estimate of the toxicity potential posed by an element from direct ingestion or dermal absorption routes, which can be calculated using the relation:
where RfDingestion/dermal represents oral/dermal reference dose (μg/kg/day) [32,33]) of each contaminant under consideration. In general, RfD is an approximate estimate of daily exposure to the human (including sensitive subgroups) that is likely to have any noticeable risk of harmful effects during a lifetime [32,33]. The RfD estimate may have an uncertainty spanning perhaps an order of magnitude.
The hazard index (HI) is the overall potential for non-carcinogenic effects posed by more than one contaminants via ingestion or dermal pathway, which can be estimated from the relation:
Carcinogenic risk (CR) associated with the ingestion pathway can be estimated using the formula:
where CSFingestion represents the cancer slope factor. Of the selected six heavy metals, Cd and Cr have significant cancer risk.
Results and discussion
The pH of water samples from Kolleru lake are near neutral to alkaline (pH varying from 6.8 to 8.8; Table 1). The TDS in these waters are variable ranging from 1006 to 5285 mg/L. Such high TDS contents indicate brackish water that has typical TDS contents of 1000 mg/L or more [37]. In a previous study, it was demonstrated that there exists a two-component mixing relationship between Na+ content of lake water and δ18O composition of the carbonate fraction of surface sediments of the Kolleru lake [24]. Two-component mixing trend was also evident from cross-plot between log(TDS) and δ18O values. Taken together, such mixing trends are indicative of influx of significant amounts of seawater into the lake [24]. Several human-induced activities in and around the lake have led to almost near stagnant to dry condition of the lake with reduced inflow and outflow, overexploitation of groundwater surrounding the lake area and breached distributaries in Upputeru River, ultimately facilitating significant intrusion of seawater [24].
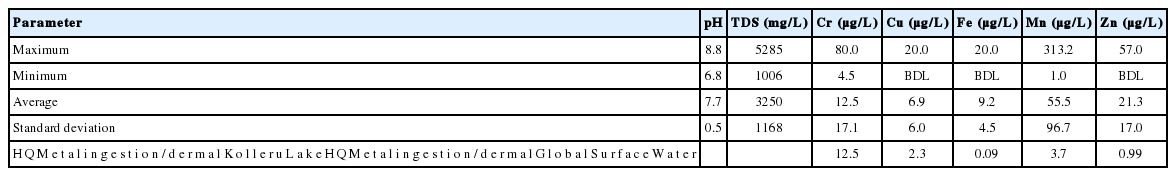
Range, average and standard deviation of various parameters, pH, total dissolved solids (TDS), and concentrations of five trace metals, chromium (Cr), copper (Cu), iron (Fe), manganese (Mn) and zinc (Zn), in water samples from the Kolleru lake, India. Cadmium (Cd) could not be estimated, as it was below detection limit (BDL) in each sample. In the last row, the average hazard quotient (HQ) estimated for the trace metals dissolved in Kolleru lake water is compared with similar data estimated for uncontaminated global surface water for which the heavy metal contents were reported in Langmuir et al. [38]
Heavy metal distribution in Kolleru lake
The range, average and standard deviation of the concentrations of trace metals Cr, Cu, Fe, Mn and Zn in the water samples of Kolleru lake are presented in Table 1. The concentration of Cd was found to be below detection limit for each sample, so I refrain from any further discussion on Cd in the later part. It is important to mention that of all the water samples #7 is characterized by maximum concentrations of Cu and Cr, although the Fe concentration of this sample is below the detection limit. Hence this particular site (Figure 1) is anomalous.
The normalized concentration ratio of five heavy metals is presented in Figure 2. The normalization is carried out with respect to the median values of concentration of these toxic elements that were reported for clean and uncontaminated surface waters (data from Langmuir [38]). Values in excess of 1 indicate enrichment and those below 1 specify depletion. It can be seen from Figure 2 that Cr is enriched and Fe is depleted, while the other elements like Cu, Mn and Zn show both enrichment as well as depletion at various locations (Figures 1 and 2). Figure 2 points to the fact that the distribution of the analyzed elements in water of the lake is highly heterogeneous, indicating that both free flow and internal mixing of water in the lake is obstructed.
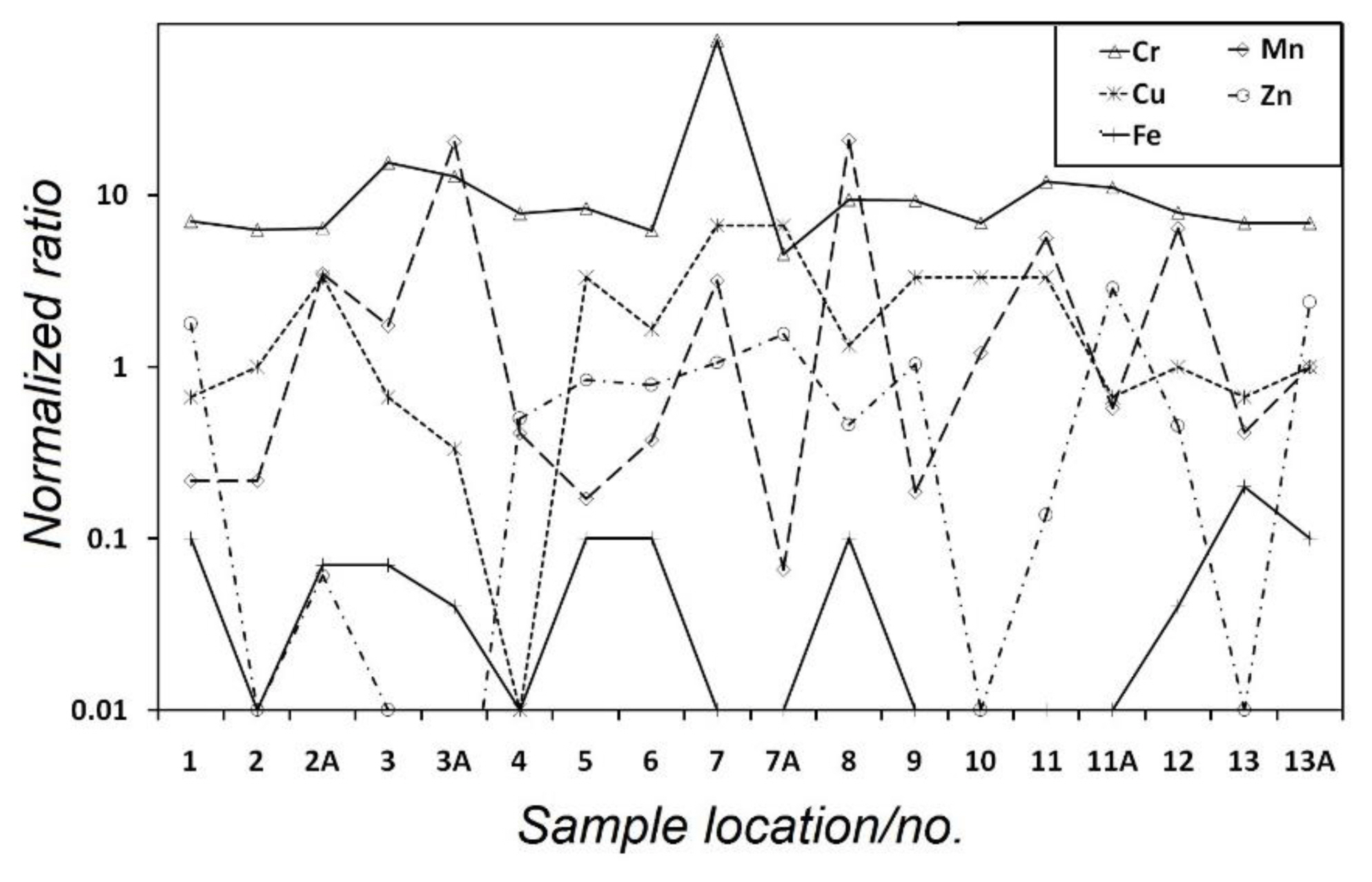
Plot showing heavy metal contaminated water chemistry of the Kolleru lake. The normalized ratios of selected toxic trace metals in the water samples of the lake are plotted for individual sampling site. For normalization, data from Langmuir [38] is used. Note that values greater than 1 indicate enrichment and those lower than 1 specify depletion.
As pointed out above, there are several anthropogenic activities which include unrestrained use of fertilizers and pesticides, fishpond discharges containing high concentrations of pesticides, polycyclic aromatic hydrocarbon (PAH) and heavy metals as indicated from the presence of such contaminants documented in the prawns cultivated in the area, discharge of industrial effluents and agricultural run-off carrying inorganic contaminants [27]. Further, 67 drains varying in length from 0.4 km to 280 km discharges water into the Lake [27]. Therefore, it is very difficult to pinpoint the exact source/sources that could be responsible for the observed high concentration in sample #7.
Non-carcinogenic health risk assessment
Health risk assessment has been carried out to explore the effect of heavy metals due to ingestion as well as dermal pathways on adults and children. Non-carcinogenic HQ values pertaining to each heavy metal for adults and children are presented in Figures 3 and 4.

Non-carcinogenic hazard quotient through ingestion pathway (HQingestion) of heavy metals, (a) chromium (Cr), (b) copper (Cu), (c) iron (Fe), (d) manganese (Mn) and (e) zinc (Zn), of the Kolleru lake. HQingestion for individual sampling site corresponding to adults, children and the respective averages are shown.
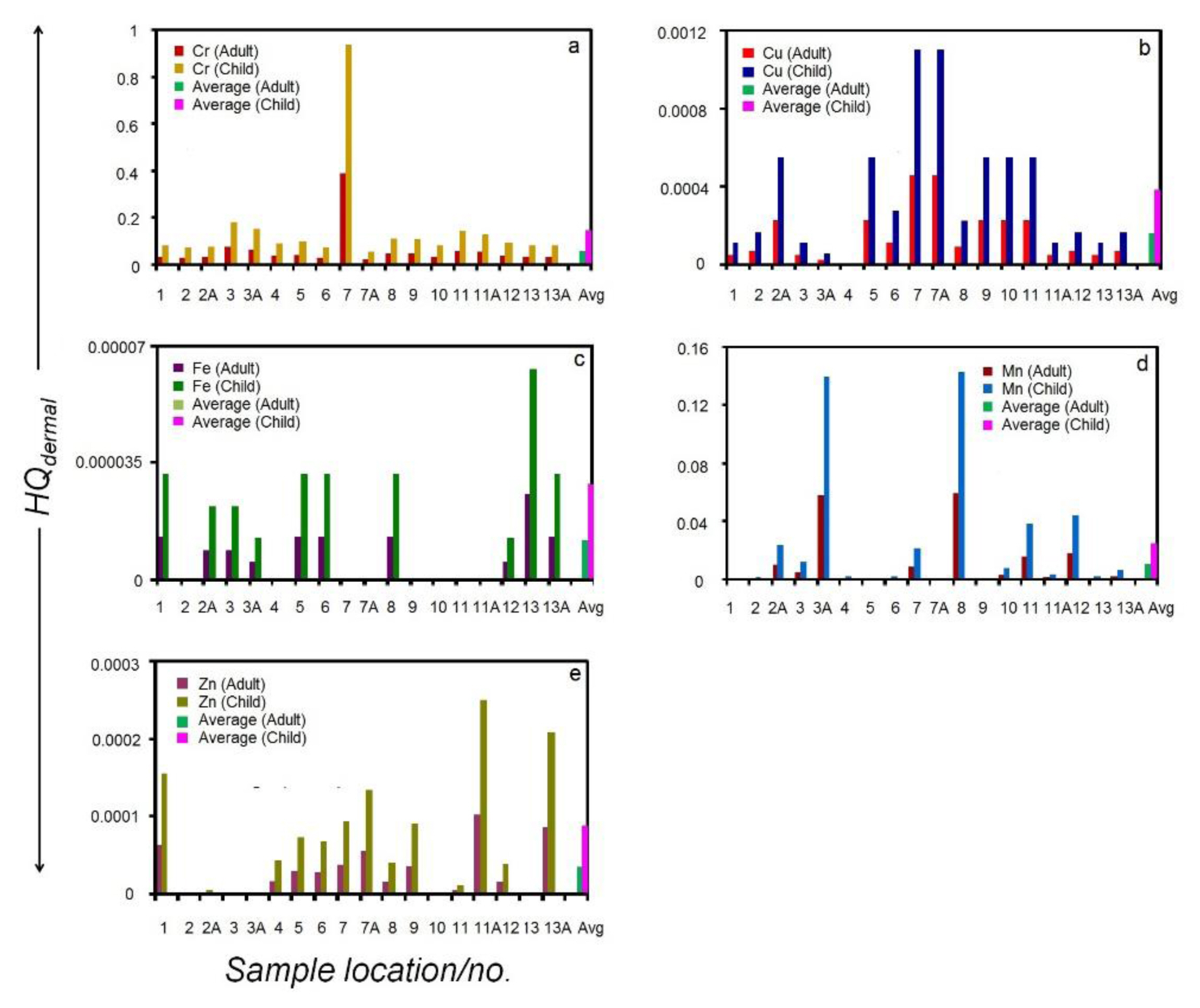
Non-carcinogenic hazard quotient through dermal pathway (HQderaml) of heavy metals, (a) chromium (Cr), (b) copper (Cu), (c) iron (Fe), (d) manganese (Mn) and (e) zinc (Zn), of the Kolleru lake. HQingestion for individual sampling site corresponding to adults, children and the respective averages are shown.
Oral pathway and dermal pathways
CDIingestion and HQingestion values were estimated using equations (1) and (3). The RfDingestion values used for each heavy metal are as follows: Cr = 3 μg/kg/day [39], Cu = 40 μg/kg/day [40], Fe = 700 μg/kg/day [41], Mn = 140 μg/kg/day [42] and Zn = 300 μg/kg/day [43].
Figure 3 shows that for both adults and children the average HQingestion values exhibit the following order: Fe < Zn < Cu < Mn < Cr. For adults, the HQingestion values for chromium show a wide range (0.093 to 1.636) with a mean value of 0.256. In the case of children, a wide range (0.133 to 2.346) is also characteristic with a mean value of 0.368. Only one sample is characterized by HQingestion value, which is greater than 1 (sample # 7, Figure 3a). The HQingestion values for Cu, Fe, Mn and Zn (Figure 3b to 3e) exhibit respectively the following ranges in the case of adults: (0 to 0.03; mean = 0.01), (0 to 0.002; mean = 0.0008), (0.0004 to 0.137; mean = 0.024) and (0 to 0.012; mean = 0.004). For children, these ranges are: Cu = 0 to 0.044, Fe = 0 to 0.0025, Mn = 0.0006 to 0.197 and Zn = 0 to 0.017 with respective mean values of 0.015, 0.0012, 0.035 and 0.006 (Figure 3b to 3e). Therefore, with the exception of one site where high Cr concentration is observed (sample # 7), the HQingestion of lake water corresponding to each heavy metal is estimated to be less than 1 (Figure 3). Further, the HQingestion values in the case of children are always high compared to those of the adults (Figure 3).
CDIdermal and HQdermal values were estimated using Equations (2) and (3) , respectively. The dermal permeability coefficient (Kp) values used in equation (2) for Cr, Cu, Fe, Mn and Zn were reported earlier, which are 2.0E-03, 1.0E-03, 1.0E-03, 1.0E-03 and 6.0E-04, respectively [33]. The RfDdermal values used in Equation (3) corresponding to each heavy metal were taken from the literature [44].
The estimated HQdermal values corresponding to five heavy metals are displayed in Figure 4. As can be seen in the diagram, the average HQdermal is characterized by values that are less than 1. When the spatial distribution of samples within the lake is considered, it is observed that the individual HQdermal values corresponding to each metal exhibit wide scatter (Figure 4a to 4e). Another interesting feature that emerges from Figure 4 is that the HQdermal values in the case of children are almost double when compared to those of adults.
Summarizing the results on estimation of HQ values due to direct ingestion and dermal absorption routes, it can be inferred that with the exception of one site where high Cr concentration is observed (sample #7), there is negligible potential risk of either direct intake or other use of lake water that is contaminated with dissolved heavy metals.
Exposures to multiple chemicals may contribute to increased health risks. Therefore, a measure of cumulative risk, HI is estimated. HI represents the overall potential for non-carcinogenic effects posed by more than one contaminants via ingestion or dermal pathway, which can be estimated using equation (4). Figure 5a and 5b exhibit the HIingestion and HIdermal values corresponding to each site of the lake. Similar to Figure 3 and 4, we observe HIingestion and HIdermal that are characterized by low values (<1), indicating negligible health risk. However, one site is characterized by high HIingestion value (Figure 5a).

Estimated non-carcinogenic hazard index (HI) and carcinogenic risk due to chromium (Cr) ingestion. The bar diagram of hazard index corresponding to adults, children and the respective averages are shown. The HI represents the overall potential for non-carcinogenic effects posed by more than one contaminant via (a) ingestion (HIingestion) and (b) dermal (HIdermal) routes. Histogram plots showing (c) carcinogenic risk due to chromium ingestion (CRCr ingestion) and (d) comparative study between average Kolleru lake water HIingestion/dermal values and those estimated for global surface water (GSW; [38]).
Carcinogenic health risk assessment due to chromium (Cr) ingestion
CRingestion of Cr was estimated using equations (1) and (5). The cancer slope factor (CSFingestion) for Cr is 500 μg/kg/day [45]. Figure 5c shows the histogram plot of estimated CRingestion of Cr for adults and children. The CRingestion values range from 0.00056 to 0.0098 with an average of 0.00154 for adults. In the case of children, the minimum (0.0008) maximum (0.0141) and average (0.0022) values are higher compared to the adults (Figure 5c). The estimated CRingestion value is indicative of the incremental probability of an individual developing cancer over a lifetime. For example, CRingestion of 10−4 indicates a probability of 1 in 10,000 individuals developing cancer [32]. Incidentally, according to the USEPA the permissible levels of total carcinogenic health risk is also 10−4 [46]. The average CRingestion levels of the adults and children are 0.00154 and 0.0022, respectively. Both these values are therefore higher than the permissible levels [46]. Furthermore, children are more vulnerable to Cr ingestion.
Comparison of health risk associated with Kolleru lake water vis-a-vis global surface water
The non-carcinogenic health risk assessment of Kolleru lake water has indicated negligible potential risk via ingestion and dermal routes. However, in the backdrop of Figure 2 shown above, it is important to estimate the health risk associated with global surface water for which the heavy metal contents were reported in Langmuir [38] and compare them with the Kolleru lake water. Table 1 presents the ratio between average HQ values associated with various heavy metals dissolved in Kolleru lake water and global surface water. It can be seen from Table 1 that three heavy metals, Cr, Mn and Cu are characterized by ratios that are greater than 1 and the ratios follow the order:
Therefore, Cr is the major contaminant in the Kolleru lake water, which is also demonstrated in this study through estimation of carcinogenic health risk assessment due to Cr ingestion. Two other metals, Zn and Fe, represent average HQ ratios that are either close to 1 (Zn = 0.99) or very less (Fe = 0.09).
The comparison study made here on the calculated HI is extremely significant. An overall insight as to how the Kolleru lake water must have been affected due to presence of dissolved heavy metals as a consequence of anthropogenic activities was plotted in Figure 5d. In this diagram the estimated HIaverage values for adults and children via ingestion and dermal pathways are displayed (Figure 5d). The HI values are also estimated based on median values of concentration of the same toxic elements [38] reported for global clean and uncontaminated surface waters (Figure 5d). These latter values are also displayed against HIaverage of Kolleru lake water for comparison. Marked differences can be noticed between the two sets of HI values (Figure 5d), indicating the degradation of water quality of the lake.
It is significant to mention that presence of various types of macrophytes such as Eichhornia crassipes, Pennisetum purpureum, Salvania sp., Ipomea aquatica and many others have been noticed within the Kolleru lake during the field survey. The toxic metal removal potential of water hyacinth was evaluated by Stephenson et al. [47] It was found by these researchers that E. crassipes can be used as remediator plants for several toxic metals like Cd, Co, Cr, Cu, Fe, Pb etc. Likewise, to improve the water quality, the role of macrophytes by preferentially scavenging different heavy metals was also reviewed [48]. In view of the above two studies, it is inferred that the estimated HQ values pertaining to various heavy metals in water of the Kolleru lake may be considered as the minimum limit. The heterogeneous distribution of HQ values displayed in Figure 3 and 4 explains the metal scavenging role of macrophytes. Although with the available data it is difficult to point out the exact source/sources and assess the relative contribution of each source for the observed heterogeneous distribution of HQ values in the lake water, it is certain that unchecked anthropogenic activities in and around the Kolleru lake area are responsible for the degradation of its water quality. In this context it is pertinent to mention that the Andhra Pradesh Government initiated a task called “Operation Kolleru” during 2005–2006. The main aim of this task was to ensure traditional activities only and to do away with all encroachments within the designated site of the lake so that free flow of water is restored. After the operation was over, migratory birds started returning to the lake, which caught attention of many. Subsequent publications also reported retrieval of the lake ecosystem [49,50]. However, recent studies reveal that several anthropogenic activities have once again picked up momentum within the lake and its surroundings [23,24,51]. Despite negligible potential non-carcinogenic health risk of either direct intake or other uses of lake water as indicated by <1 HQ and HI values (Figure 3, 4, and 5), this study demonstrates that the ratios between estimated average HQ values associated with various heavy metals like Cr, Cu and Mn dissolved in Kolleru lake water and global surface water is high (Table 1 and Figure 5c and 5d). Therefore, there is an urgent need to stop further degradation of the lake and its surroundings. In order to ensure sustenance of the lake’s health, concerted efforts are needed on local and regional scales.
Conclusion and recommendation
The individual HQingestion HQdermal values are estimated to be less than 1 for the heavy metals dissolved in Kolleru lake water. Such low values are indicative of an acceptable level of non-carcinogenic health risk for the heavy metals dissolved in water.
The average HIingestion and HIdermal, for both adults and children are also less than 1, suggesting negligible amount of overall adverse health risk.
Water intake represents only a proportion of fluid consumed. However, taking other food items such as fish of the lake and rice grown in the lake surroundings when consumed, can have adverse health effect. Because in such case, cumulative ingestion candidates may contribute to intake of increased amounts of heavy metals.
As far as average CRingestion levels of the adults and children due to Cr are concerned, high CRingestion values of 0.00154 and 0.0022, respectively are estimated for adults and children. Both these values are higher than the permissible levels [46].
It is therefore recommended that monitoring the levels of heavy metal in water and other items like fish of the lake, rice and vegetables grown in the area need to be carried out at regular intervals.
In view of the phytoremediation potential of various species discussed in the literature [47,48], one of the most pertinent questions that may arise is whether to eliminate or maintain the macrophytes in the lake ecosystem. This is particularly important in view of sampling site #7, where anomalously high concentration of Cr is registered. As a mitigation measure, it is therefore suggested that the phytoextraction process may be continued for some more time, even if it is at the expense of restriction of free flow of water in the lake. However, once the phytoextraction processes are over, the macrophyte species need to be disposed safely during the initial stages of restoration phase of the lake. Subsequently, if the domestic and industrial effluents are allowed to discharge into the lake, proper treatment becomes mandatory to ensure no toxicity.
The natural macrophytes that preferentially scavenge heavy metals and present in the lake need to be disposed periodically after the phytoextraction processes. In this context, strategies may be made about their safe disposal. For example, Sas-Nowosielska et al. [52] examined a large number of strategies that include composting, compaction, incineration, ashing, pyrolysis, direct disposal, liquid extraction, etc. They suggested that incineration (smelting) is the most feasible, economically viable and environmentally friendly method. However, there are other researchers who proposed that pyrolysis of hyperaccumulator biomass is the most suitable method [53,54]. According to them, the char can be considered a rich “ore” or metal concentrate, which can be processed for possible separation of the metal in an ore-processing unit. Such mitigation measures could prove to be extremely beneficial for long-term sustenance of the lake ecosystem.
The results presented in this study and recommendation made thereof is expected to provide adequate insight to the local government and health professionals to evolve strategies to effectively manage and mitigate the water quality of the Kolleru lake.
Acknowledgement
I am grateful to Jung-Hwan Kwon, Editor-in-Chief for efficient handling and useful suggestions. Two anonymous reviewers are acknowledged for critical evaluation of the manuscript. Suggestions made by them have helped in improving the manuscript.
Notes
Conflict of interest
The author declares no conflict of interest associated with the material presented in this paper.
