Prevalence of antibiotic-resistant, toxic metal-tolerant and biofilm-forming bacteria in hospital surroundings
Article information
Abstract
The emergence and rapid spread of antibiotic-resistant bacteria due to unethical and non-scientific disposal of hospital wastes and clinical by-products caused an alarming environmental concern and associated public health risks. The present study aims to assess the co-selection of antibiotic resistance and heavy metal tolerance by bacteria isolated from hospital effluents. These isolates were also tested for hemolytic activity, pH-tolerance, thermal inactivation, auto-aggregation, cell-surface hydrophobicity and interaction with other bacteria. The study reports the prevalence of antibiotic-resistant and heavy metal tolerant bacteria in clinical effluents and water samples. Most of these isolates were resistant to vancomycin, clindamycin, ampicillin, rifampicin, penicillin-G, methicillin and cefdinir, and evidenced the production of extended-spectrum β-lactamase enzyme. Toxic metals such as cadmium, copper, iron, lead and zinc also exert a selection pressure towards antibiotic resistance. Pseudomonas aeruginosa strain GCC_19W3, Bacillus sp. strain GCC_19S2 and Achromobacter spanius strain GCC_SB1 showed β-hemolysis, evidenced by the complete breakdown of the red blood cells. Highest auto-aggregation was exhibited by Bacillus sp. strain GCC_19S2; whereas, maximum cell-surface hydrophobicity was displayed by P. aeruginosa strain GCC_19W1. Antagonistic activity by Stenotrophomonas maltophilia strain GCC_19W2, P. aeruginosa strain GCC_19W3 and strains of Achromobacter restricts the growth of other microorganisms by producing some bactericidal substances. The study emphasises undertaking safety measures for the disposal of clinical effluents directly into the environment. The study suggests adopting necessary measures and regulations to restrict the spread of emerging pathogens within the hospital biome and community, which if unnoticed, might pose a significant clinical challenge.
Introduction
In the environment, antibiotic-resistance emerges in water, soil and sediments through contamination with human and animal wastes that contain antibiotic-resistant bacteria [1]. The discharge of inadequately treated or untreated wastewater from hospitals significantly contributes to the threat of antibiotic resistance [2]. Hospitals use a variety of strategies to protect the patients, but little has been done to safeguard the environment from the contamination due to clinical effluents. The clinical effluents include the wastewater and solid wastes generated from all the medical and non-medical activities. Hospital wastes refer to the infectious, non-hazardous and hazardous liquid waste with sufficient free liquid arising from dental, medical, nursing, pharmaceutical or similar practices [3]. These wastes can be hazardous, toxic and even lethal, because of their high potential for transmitting diseases [4]. Hospital bacterial ecological niches intersect with those in the environment, resulting in public health risks.
Pseudomonas aeruginosa and Bacillus sp. are ubiquitous in hospital wastewater discharges. The infections caused by P. aeruginosa become complicated, as they acquire the ability to resist many classes of antibiotics. Some strains of P. aeruginosa has been reported to survive for months on dry surfaces, and it can persist and grow in contaminated antimicrobial handwash containing triclosan, making it a critical concern for hospital staff [5]. Infections with Bacillus sp. are rare, but they are able to produce spores and grow haemolytically on blood agar plates (β-hemolysis). Gram-positive Bacilli can cause many types of infections and are spread to humans in a variety of ways [6,7]. Some of the frequently encountered species include B. subtilis, B. cereus, B. licheniformis, B. anthracis, B. megaterium, and B. pumilus. Bacillus sp. may cause ocular infections including conjunctivitis, endophthalmitis, keratitis, dacryocystitis, panophthalmitis and iridocyclitis [8]. In 2019, a rare case of bloodstream infection with Bacillus mycoides in human have been reported in hospital settings of the Netherlands [6]. Stenotrophomonas maltophilia has emerged as an important nosocomial pathogen associated with a variety of infections, such as infection of the bloodstream, respiratory tract, urinary tract, bone and joint, endocarditis and meningitis [9]. It is one of the leading opportunistic multidrug-resistance pathogens in hospitals worldwide. The genus Achromobacter is an emerging nosocomial pathogen, which the most frequently found in contaminated solutions of dialysis water, demineralized water, water from nebulizers, humidifiers, incubators, extracorporeal circulation systems, poorly preserved heparin flasks and even antiseptic and disinfectant solutions [10,11]. The gram-negative Cedecea davisae bacterium has been implicated in causing infection in skin, soft tissue and lung, and in rare cases, they are associated with catheter-related bloodstream infections [12]. Though infections with C. davisae or the genus Cedecea are rare, treating them is a challenge due to their broad-spectrum antibiotic resistance [13]. The synthesis of extended-spectrum-β-lactamase (ESBL) enzyme is one of the mechanisms by which different bacteria develop antibiotic resistance. These enzymes hydrolyse the β-lactam ring structure of the antibiotic, thereby rendering it inactive [14]. The most common bacteria found to possess ESBL genes include Escherichia coli [15], Klebsiella pneumoniae [16], P. aeruginosa [17], Bacillus sp. [18], Shigella sp. and Salmonella sp., [19]. Hospital effluents containing various chemical agents, such as antibiotics, toxic heavy metals etc., interact with diverse microbial agents including pathogenic bacteria and lead to an acquisition of selective pressure [20]. The relationship between microbial acquisition of antibiotic resistance and metal tolerance has been extensively studied by many researchers, and both of these resistance genes are found to be located on the same mobile genetic elements [21,22].
It is evident that the resistant bacterial strains gain entrance to the environment via the indiscriminate discharge of untreated or partially treated wastewater from domestic, clinical and industrial sources [14]. To formulate effective practices and policies for combating antibiotic-resistance and to prevent the direct exposure of pathogenic microbes into the environment, it is essential to investigate the biofilm composition, their distribution, degree of antibiotic resistance, and their growth kinetics with change in pH and temperature. Bacteria generally protect itself from external stress such as nutrient starvation or oxidative stress by auto-aggregation mechanism [23]. This property also plays an important role in protection from the host immune system [24]. The molecular mechanisms of bacterial auto-aggregation in aqueous solution may depend on hydrophobic surface properties [25]. The hydrophobic properties favor adhesion to biotic and abiotic surfaces and penetration of host tissues [26]. The efficiency of adhesion, aggregation and biofilm formation by bacteria discharged from hospitals must be evaluated to prevent or reduce the risk of disease transmission and serious damage to the environment.
The bacteria present in hospital effluents regularly interact with the environment, either by synergism or by antagonism relationships [27]. Microorganisms produce antimicrobial substances such as bacteriocin, antibiotic or other toxic components that can directly restrict the growth of other microorganisms. Hussein et al. [28] isolated antibiotic-producing Actinobacteria from the sediment of Ma’in thermal springs (Jordan), which showed an apparent inhibitory activity against the strains of E. coli, P. aeruginosa, B. cereus and S. aureus. Many classes of antimicrobial substances have been identified and reported in the literature [29–31].
The present study aims to assess the co-selection of antibiotic resistance and heavy metal tolerance by bacteria isolated from hospital effluents. The ability of these isolates to produce ESBL was evaluated, and their hemolytic activity was also studied. These isolates were also tested for pH tolerance, thermal inactivation, auto-aggregation, cell-surface hydrophobicity and their interaction with other bacteria.
Materials and Methods
Isolation and Characterization of Isolates
Collection of samples
Clinical effluents were collected from four different hospital premises in Silchar, which is situated in Cachar district, in the state of Assam, India (Figure. 1). The sampling sites were drainage area, sewer manhole and clinical garbage dumping site, from where 17 samples were collected in sterile containers and immediately brought to the laboratory for further studies. Drinking water samples were also collected from two hospitals and three nearby tea stalls, to assess the transmission of pathogenic bacteria.
Isolation and characterization of bacteria
Isolation and quantitative computation of bacteria from effluents samples were performed by serial dilution techniques [32]. 100 μl of samples were spread onto nutrient agar (NA) plates and incubated at 37 °C for 24 hou9rs. The total bacterial count was determined by counting the colonies in microprocessor colony counter. Individual distinct colonies were sub-cultured to get pure isolates. Isolates were further identified by colony morphology (shape, structure, colour, pattern and size), Gram’s staining and biochemical tests. Biochemical tests include indole production test, methyl red (MR) test, Voges-Proskauer (VP) test, citrate utilisation test, starch hydrolysis test, catalase activity, oxidase activity and Triple-sugar iron (TSI) test [33].
Molecular identification
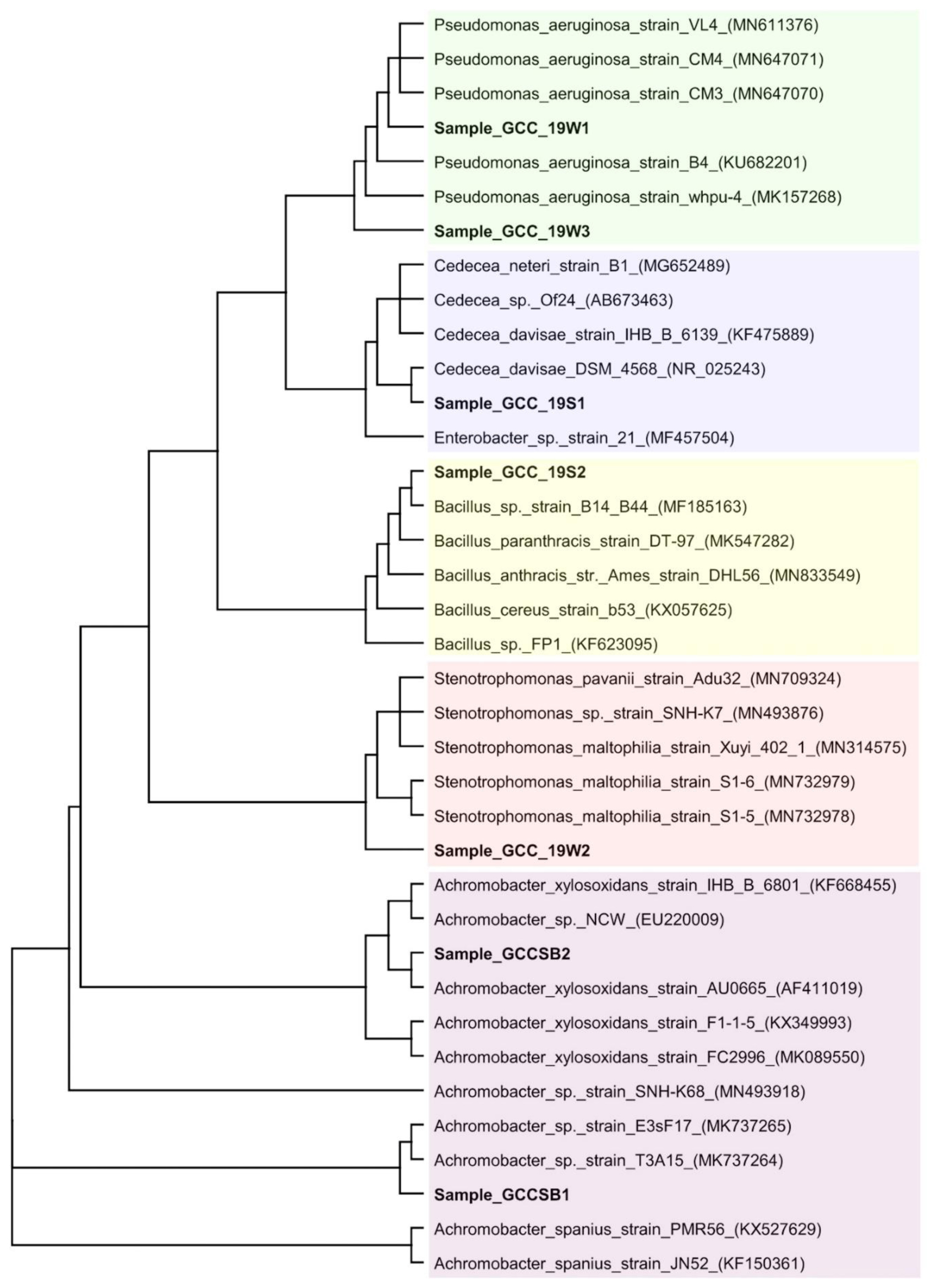
Genomic DNA was extracted from isolated bacterial cultures [34] and PCR amplification of 16S rDNA gene was achieved by 704F (5′-GTAGCGGTGAAATGCGTAGA-3′) and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′) [35]. Sequencing of 16S rDNA was carried out at Xcelris Labs Limited, Gujarat, India using ABI 3730xl 96 capillary system using Big Dye Terminator v3.1 kit. The consensus sequence was generated from forward and reverse sequence data, which was used to perform BLAST search. Based on the maximum identity score, first ten sequences were selected, aligned using Clustal-W and a phylogenetic tree was constructed using FastTree [36]. FastTree algorithm improves the topology and the branch lengths of the phylogenetic tree, with maximum-likelihood rearrangements. Geneious R8 software package (Biomatters Ltd., Auckland, New Zealand) was used to perform all the molecular analysis.
Tests concerning the resistance of the strains towards environmental conditions
Determination of optimum pH
NA plates were prepared with varying pH, ranging from pH 2 to pH 8. The test isolates were streaked onto the plates and incubated at 37 °C for 24 hours. Their growth pattern at extreme acidic and basic environment was recorded, and optimum pH was noted.
Determination of thermal death time
Thermal death time at 60 °C and 100 °C was determined by incubating the broth cultures in a temperature-controlled water bath for 1 hour [37]. After every 10 mins, bacterial samples were streaked on freshly prepared NA plates and incubated for 24 hours at 37 °C.
Test for heavy metal tolerance
Bacterial tolerance to heavy metals (Cadmium (Cd), Copper (Cu), Iron (Fe), Lead (Pb) and Zinc (Zn)) was determined by agar dilution method, which was added in the form of cadmium sulfate, copper (II) sulfate, ferrous sulfate, lead acetate and zinc powder [38–40]. The initial concentration of these metal salts in nutrient plates was 100 μg/mL, and bacterial growth was observed by streaking on respective plates. Metal concentration was progressively increased by 50–100 μg/mL on a fresh agar plate, and the minimum inhibitory concentration (MIC) was noted when the isolates failed to grow on respective plates [41]. The experiment was conducted separately for Cd, Cu, Fe, Pb and Zn taking five replicates at each concentration.
Test concerning putative pathogenicity traits
Hemolysis test
Freshly cultured bacteria were streaked on blood agar plates containing 5% (v/v) sheep blood and incubated at 30 °C for 24 hours. The blood agar plate was examined for signs of β-hemolysis (clear zone around the colonies), α-hemolysis (green-hued zones around colonies) or γ-hemolysis (no zones around the colonies) [42].
Auto-aggregation assay
Auto-aggregation assays were performed by following the protocol of Basson et al. [43] with minor modifications. The overnight grown bacterial broth was centrifuged at 10,000 rpm for 5 mins at 4 °C, and pallets were collected. Pellets were washed twice by PBS buffer (pH 7.2), re-suspended in 6 mL of PBS buffer, and the initial absorbance was noted at 600 nm. To conduct the auto-aggregation assay, the cell suspension was kept undisturbed for 2 hours at room temperature. After the incubation period, 1 mL from the upper suspension was taken, and the final absorbance was measured at 600 nm. Auto-aggregation was determined by measuring absorbance at 0 hour (initial) and 2 hours (final). The auto-aggregation percentage was measured by the formula:
Cell surface hydrophobicity assay
The 20 hours old bacterial cultures were centrifuged at 12000 rpm for 5 min at 4 °C, and bacterial cell pellets were collected. Cell pellets were washed twice with PBS, and the initial optical density was recorded at 600 nm. The bacterial suspension was then mixed with 1 mL of hydrocarbons (n- Hexadecane and Toluene), vortexed for 2 mins and left undisturbed for 1 hour for phase separation. The aqueous phase was then removed carefully with a micropipette, and the final absorbance was measured at 600 nm. The percentage of cell surface hydrophobicity was determined by the formula: equation 1 [44].
Determination of antibiotic susceptibility and resistance pattern
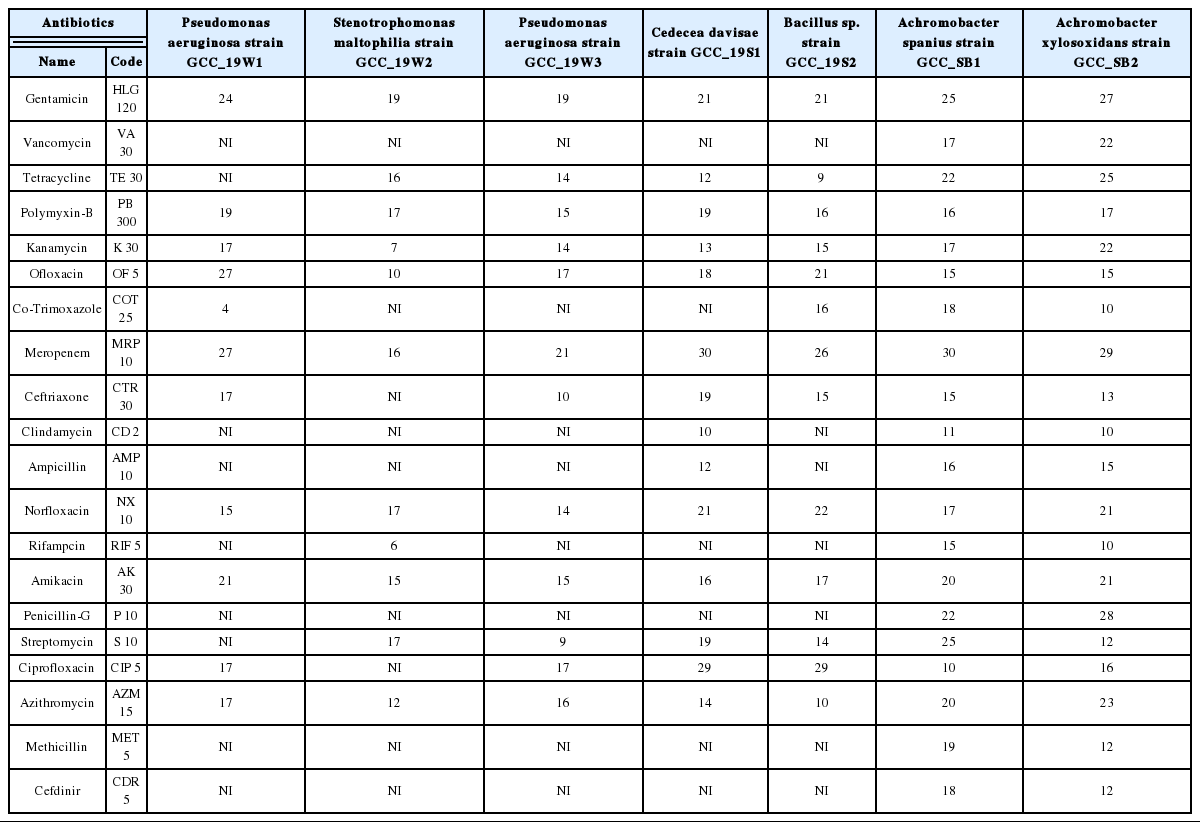
The isolates were tested against 24 antibiotics using the Kirby-Bauer disc diffusion technique [45]. Antibiotic discs were placed on freshly prepared lawns of each isolate on Mueller-Hinton agar (MHA) plates and incubated at 37 °C for 24–48 hours. The diameter of the inhibition zones was measured, and the strains were classified following the standard antibiotic disc chart. Standard antibiotic discs were procured from ‘HiMedia’ which includes gentamicin (120 μg), vancomycin (30 μg), tetracycline (30 μg), polymixin-B (300 μg), kanamycin (30 μg), ofloxacin (5 μg), co-trimoxazole (25 μg), meropenem (10 μg), ceftriaxone (30 μg), clindamycin (2 μg), ampicillin (10 μg), norfloxacin (10 μg), rifampicin (5 μg), amikacin (30 μg), penicillin-G 10μg), cefdinir (5 μg), ciprofloxacin (5 μg), azithromycin (15 μg), methicilin (5 μg), and streptomycin (10 μg).
Screening for the production of extended-spectrum β-lactamase (ESBL) enzyme
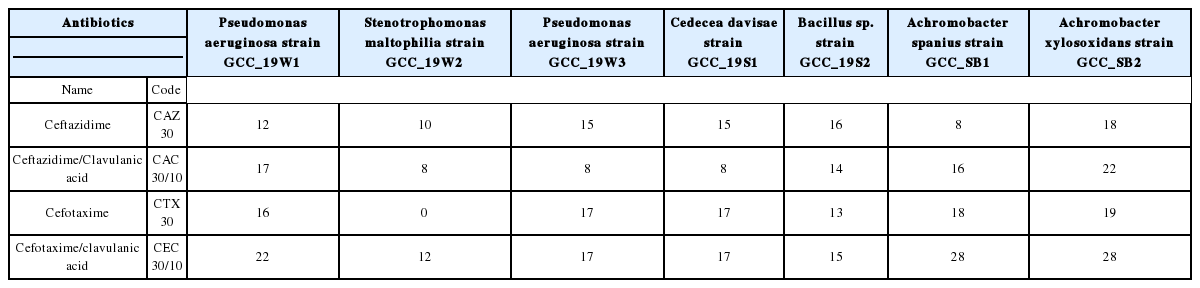
Combined disk method was performed for the phenotypic detection of ESBL producing isolates. In this test, disks containing cefotaxime (30 μg) and ceftazidime (30 μg) with and without clavulanic acid (10 μg) were used. The test isolates were inoculated on the MHA plate and the antibiotic discs with and without clavulanic acid were placed at a distance of 20 mm from each disk. The plates were incubated at 37 °C for 48 hours, and the resulting inhibition zones were measured. ESBL positive isolates were confirmed when the difference of zone diameters between the β-lactam antibiotic disk and the disk containing the antibiotic with clavulanic acid was greater than 5mm [46].
Test concerning competitiveness
Extraction of bacterial metabolites
For extraction of bacterial metabolites, 5 mL of overnight grown broth culture was mixed with an equal volume of ethyl acetate and shaken well in a rotary shaker at 20 rpm for 10 mins. Isolates were centrifuged, and the upper phase has been transferred into a new flask to evaporate ethyl acetate. Then, the remaining content was mixed with ethyl acetate: acetone: methanol (1:1:1; v/v/v), which is used to evaluate the antagonistic activity [28].
Antagonistic activity of bacterial metabolites against test pathogens
Three strains of bacteria were used as test pathogens to investigate the antagonistic activity of the Lactobacillus spp. They are Bacillus cereus strain SN_SA (MH482928), Acinetobacter johnsonii strain SB_SK (MH482927), P. aeruginosa strain GCC_19W1 (MN066610), obtained from the culture collection of Institutional Biotech Hub, Gurucharan College, Silchar.
The effectiveness of bacterial metabolites was studied by the well diffusion method [47]. MHA plates were first seeded with the respective test pathogens, and aliquots of 60 μL of the sterile cell-free supernatant were placed in 6 mm diameter wells on MHA plates. After 24 hours of incubation at 37 °C, the diameters of the zones of growth inhibition were classified as sensitive, moderate sensitive or resistant [48]. Dimethyl sulfoxide (DMSO) was used as control, and the test was performed in triplicate.
Results
Identification of bacteria
It has been observed that isolates GCC_19W1, GCC_19W2 and GCC_19W3, recovered from hospital wastewater forms white, yellow and green colonies, with a jelly-like surface on NA media. Microscopic observation showed that these isolates were gram-negative and rod-shaped. Biochemical test results demonstrated positive results for citrate test, catalase test and TSI test, whereas negative results for indole production test and VP test. Isolate GCC_19W2 showed negative results for MR test, starch hydrolysis and oxidase test (Table 1). Two isolates recovered from solid wastes (GCC_19S1 and GCC_19S2) showed different morphological and biochemical characteristics. GCC_19S1 showed dark pink colour with a jelly-like appearance in NA plates. The isolate was gram-negative rod-shaped and showed negative results for indole production test, MR test, VP test and TSI test. Isolate GCC_19S2 forms white colonies with mucus type appearance on NA plates. Microscopic observation showed the isolate as gram-positive rod-shaped, and biochemical test results evidenced positive results for VP test, catalase test and oxidase test. Drinking water samples were also collected from off-campus hospital area nearby tea stalls. The isolates recovered from hospital drinking water and nearby tea stalls were designated as GCC_SB1 and GCC_SB2 respectively, which appears to be white to light brown and jelly-like appearance in NA plates. Both of these isolates were gram-negative rod-shaped and showed negative test results for indole production, MR, starch hydrolysis and TSI (Table 1).
Molecular identification of the isolated bacteria was done by analyzing the 16S rDNA sequence. The BLAST-N algorithm finds regions of similarity between biological sequences present in the database, which demonstrated that the query sequence has 97–99% identity and 100% query coverage with the 16S rDNA of the bacterium recorded in the GenBank. Phylogenetic tree inferred the degree of relatedness between 16S rDNA sequence of the isolates with other closely related sequences retrieved from the database (Figure. 2). Based on these data, the isolates were identified as P. aeruginosa strain GCC_19W1 (GenBank accession number: MN066610), S. matophilia strain GCC_19W2 (GenBank accession number: MN066611), P. aeruginosa strain GCC_19W3 (GenBank accession number: MT050053), C. davisae strain GCC_19S1 (GenBank accession number: MN066609), Bacillus sp. strain GCC_19S2 (GenBank accession number: MN066608), Achromobacter spanius strain GCC_SB1 (GenBank accession number: MK000623) and Achromobacter xylosoxidans strain GCC_SB2 (GenBank accession number: MK000624).
Optimal pH for bacterial growth
Isolated strains of P. aeruginosa and Bacillus sp. demonstrated optimal growth at pH 7.0–7.5, and their growth in agar plates was not drastically affected with slight increase or decrease in pH. Achromobacter sp. exhibited an exponential growth at pH 7.0–8.0, whereas S. maltophilia strain GCC_19W2 and C. davisae GCC_19S1 exhibited optimal growth at pH 6.5–7.0.
Thermal death time of the isolates
None of the test isolates was able to tolerate the temperature regime of 100 °C. However, when the temperature was lowered to 60 °C, A. spanius strain GCC_SB1 showed significant growth for 70 mins. Bacillus sp. strain GCC_19S2 and strains of P. aeruginosa also demonstrated significant growth at 60 °C up to 50 mins. S. maltophilia strain GCC_19W2 and C. davisae strain GCC_19S1 showed moderate growth, demonstrating thermal death time of 20 mins at 60 °C.
Tolerance of isolated bacteria towards heavy metals
The co-selection of antibiotic resistance and heavy metal tolerance has been observed by all isolates (Table 2). Isolated strains of S. maltophilia, C. davisae, Bacillus sp. and Achromobacter sp. showed maximum growth in NA media supplemented with zinc. S. maltophilia strain GCC_19W2 showed considerable tolerance towards all tested metals, exhibiting a MIC of 3000 ± 54.77 μg/mL for cadmium, 2060 ± 67.82 μg/mL for copper, 3480 ± 58.31 μg/mL for iron and 3980 ± 66.33 μg/mL for lead in NA media. Isolates P. aeruginosa strain GCC_19W1, C. davisae strain GCC_19S1 and A. spanius strain GCC_SB1 are able to withstand 4000 μg/mL of lead in NA amended plates. A. xylosoxidans strain GCC_SB2 showed maximum tolerance towards iron with MIC of 4080 ± 48.99 μg/mL of NA. The present study reports a lower tolerance limit of P. aeruginosa strain GCC_19W1 against all the tested metal salts. Copper amendment NA plates showed the minimum growth kinetics, with MIC ranging from 1000 ± 44.72 to 2160 ± 44 μg/mL of NA.
Hemolytic activity of the isolated strains
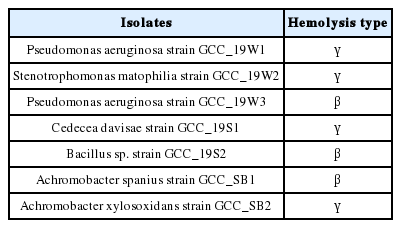
P. aeruginosa strain GCC_19W3, Bacillus sp. strain GCC_19S2 and A. spanius strain GCC_SB1 showed β-hemolysis (Table 3), which is evidenced by the complete breakdown of the red blood cells leaving a clear zone. The remaining four isolates showed γ-hemolysis, and no breakdown of the red blood cells occurred.
Auto-aggregation of bacterial isolates
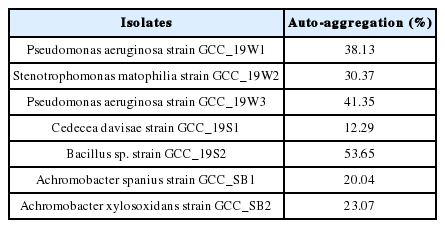
Bacteria can exhibit different auto-aggregation abilities ranging from high (50% and above), medium (35–50%), and low (16–35%) [49]. Bacillus sp. strain GCC_19S2 reported the highest auto-aggregation ability (53.65 %), followed by P. aeruginosa strain GCC_19W3 and P. aeruginosa strain GCC_19W1 with auto-aggregation percentage of 38.13 and 41.35 respectively (Table 4). S. maltophilia strain GCC_19W2 and strains of Achromobacter sp. showed a significant auto-aggregation ability to form aggregates. The least capability of auto-aggregation by C. davisae strain GCC_19S1 (12.29%) demonstrates their lower efficiency in adhesion and colonisation in the host epithelium.
Cell-surface hydrophobicity of isolates
The analysis of cell surface hydrophobicity is an essential parameter for determining the colonisation of microorganisms in the host cells [50]. Among all the isolated strains, maximum hydrophobic interaction was observed by the strains of P. aeruginosa strain GCC_19W1; having hydrophobicity values of 40.11 and 55.09 for n-hexadecane and toluene respectively. However, the percent hydrophobic index less than 70% cannot be classified as hydrophobic [51]. Therefore, from the obtained results, all the strains are classified as weak hydrophobic (Table 5).
Antibiotic resistance pattern
The gram-negative strains of P. aeruginosa and S. maltophilia showed resistance towards vancomycin, co-trimoxazole, clindamycin, ampicillin, rifampicin, penicillin-G, methicillin and cefdinir (Table 6). A similar trend was observed by Bacillus sp. strain GCC_19S2, exhibiting their ubiquitous nature, fast adaptation and acquisition of antibiotic resistance. The gram-negative C. davisae has been reported to cause infection in the skin and soft tissues, and in some cases associated with bloodstream infections [12]. The prevalence of antibiotic-resistant C. davisae outside the hospital environment is rare, but the study cues their outbreak which may cause serious challenge, if unnoticed. The strains of Achromobacter sp., isolated from drinking water, were susceptible to all tested antibiotics.
Production of extended-spectrum β-lactamase (ESBL) enzyme
The production of β-lactamase enzyme by gram-negative bacteria has tremendous therapeutic consequences, and if it remains undetected, it might pose a significant clinical challenge. The study reports the prevalence of ESBL positive bacteria by all the gram-negative bacteria (Table 7). These isolates producing the ESBL also conferred resistance towards a group of antibiotics.
Antagonistic effect of isolated bacterial metabolites on the growth of other bacteria
Antagonism is a widespread bacterial trait, resulting from the production of diffusible inhibitory or antibiotic substances [52,53]. The human opportunist pathogen S. maltophilia strain GCC_19W2 demonstrated inhibitory effect against laboratory strains of P. aeruginosa, B. cereus and Acinetobacter johnsonii with a zone of inhibition of 9 ± 0.58 mm, 8.67 ± 0.33 mm and 6.33 ± 0.33 mm respectively. P. aeruginosa strain GCC_19W3 and Achromobacter sp., recovered from wastewater and drinking water also evidenced antagonistic activity against the tested bacterial strains (Table 8). Bacillus sp. was found to be effective against A. johnsonii strain SB_SK, whereas, C. davisae strain GCC_19S1 fails to show any inhibition zone. Cross-examination by inoculating the sample from the zone of inhibition on NA plates showed no growth of lab isolates bacteria, thereby demonstrating their bactericidal activity.
DISCUSSION
In recent years, the emergence and spread of antimicrobial-resistant microorganisms have posed an increasing threat to health care. Hospital-acquired infections due to microorganisms that are resistant to multiple antibiotics have increased morbidity, mortality and the overall cost of healthcare [54]. The present study reports multi-drug resistance pattern of bacteria that may adversely affect the environment, community and sometimes leads to treatment failure. P. aeruginosa and Bacillus sp. reported in the present study are ubiquitous in hospital wastewater discharges, acquiring a wide range of resistance towards antibiotics, heavy metals and other contaminants [55–57]. The occurrence of C. davisae in healthcare-associated infections has also been reported by many researchers [13,12].
The growth of these microbes can be greatly affected by the change in temperature and pH of the environment, and this principle has been adopted as a method of disinfection. It has been observed that, at a temperature regime 100 °C, none of these isolates were able to survive. On the other hand, when the temperature was lowered to 60°C, A. spanius strain GCC_SB1, Bacillus sp. strain GCC_19S2 and P. aeruginosa showed significant growth for 50 mins. Bacterial thermal tolerance can be affected by various factors such as growth temperature [58], pH [59], water activity [60] and growth medium [61]. All the isolates showed varied optimal pH requirements ranging from pH 6–8, whereas extreme acidic and basic pH significantly decreases the bacterial growth pattern. The relation between pH and the bacteriostatic effect has been studied by many researchers [62,63]. Upon entering the cell, the organic acids lower the cytoplasmic pH, resulting in osmotic stress and partial collapse of the transmembrane proton gradient [64,65]. The result of the present study provides an insight into planning successful composting strategies for potentially hazardous contaminants and biological agents. Moreover, proper disposal of hospital effluents should be performed, excessive usage of antibiotics and other chemicals should be restricted, and drinking water must be boiled to restrict or kill any harmful bacteria. Hospital waste contains a large amount of heavy metals such as chromium, cadmium, lead, mercury, zinc etc., as well as organic compounds. These contaminants pollute the environment and pose public health risks [66,67]. In some cases, hospital wastes are treated by incineration, which involves the combustion of organic substances that are contained in waste materials. However, they cannot destroy the metallic components of the waste and concentrate heavy metals into the bottom ash [68].
The virulence nature of these isolates was further accessed by the production of hemolysin. Among all, P. aeruginosa strain GCC_19W3, Bacillus sp. strain GCC_19S2 and A. spanius strain GCC_SB1 showed β-hemolysis, evidenced by the complete breakdown of the red blood cells leaving a clear zone. Hemolysin production among gram-negative bacteria is an indicator of the virulence factors [69]. The ability of auto-aggregation to form aggregates also provides a gateway to colonise to the biotic and abiotic surface and successive penetration to host tissue. Both environmental and pathogenic bacteria exhibit the property of auto-aggregation, generally mediated by self-recognising surface structures, such as proteins and exopolysaccharides [70]. The present study reports moderate auto-aggregation by the strains of Pseudomonas and Bacillus, might help in adapting to antibiotics, heavy metals and other environmental contaminants. Depending on the bacteria, the auto-aggregative phenotype may be constitutive or induced under certain conditions, such as stress, oxygen availability or a change in temperature [25]. Bacterial pathogenesis involves the initial adhesion of the bacteria to the intestinal epithelium in the ileum, followed by colonisation of the colon and production of toxin. The toxin impairs epithelial barrier function and ion transport, causing diarrhea [71]. The least capability of auto-aggregation (12.29%) by C. davisae strain GCC_19S1 demonstrates their lower efficiency in adhesion and colonisation in the host epithelium. Analysis of cell surface hydrophobicity is an also essential parameter for determining the colonisation of microorganisms in the host cells [50]. Among all the isolated strains, maximum hydrophobic interaction was shown by the strains of P. aeruginosa. Similar studies were conducted by Fiorina et al. [72], who isolated bacteria from biofilms of water distribution pipes and colonised catheters from hospitalized patients. Cell surface hydrophobicity is a major parameter for controlling the adhesion of bacteria to surgical tools, medical implants, and also within the environment [73–75]. It also plays an important role in fouling, transmission of infections, and contamination of fruits and vegetables [76,77].
The present study reports the co-selection of antibiotic resistance and heavy metal tolerance by all isolates. The physical linkage of antibiotic resistance and metal resistance is encoded on the plasmid that confers resistance to the bacteria even when only one co-selecting agent (either antibiotics or heavy metals) is present [78–80]. Co-selection of antibiotic resistance and heavy metal tolerance by P. aeruginosa is well documented in many studies [81,82,41,83]. Bacillus sp. and Stenotrophomonas sp. responds to a wide range of environmental contaminants, and exerts co-selection of heavy metal tolerance and antibiotic resistance [41,84,85]. Multidrug-resistant C. davisae strain GCC_19S1 reported in the present study showed tolerance to a group of toxic metals. A study conducted by Resende et al. [86] demonstrated that clinical isolates of C. davisae exhibiting tolerance towards Ni, Zn, Cr, Cu, Cd and Hg, acquires a selection pressure towards a group of antibiotics. In another study, Istiaq et al. [87] reported that strains of A. xylosoxidans possess genes for Arsenic (As), Cu, Zn, and Cd resistance, which also display a broad range of resistance against antibiotics belonging to β-lactams, aminoglycosides, cephalosporins, monobactams and even macrolides. Genomic investigation of S. maltophilia SJTH1 isolated from the hospitalized patient of PGIMER, Chandigarh, India revealed diverse antibiotic resistance genes, efflux pumps, heavy metal resistance, various transcriptional regulators etc. [88]. Reports are also available on multidrug-resistant C. davisae from Indian Hospital [89]. S. matophilia and Achromobacter xylosoxidans have been reported as an emerging nosocomial, multidrug-resistant, opportunistic pathogen in hospital settings, which is also evident in the present study [90–92]. The antibiotics of β-lactam and carbapenems group are the common antibiotics which are used to treat the infections caused by Gram-negative group of bacterial pathogens. Surprisingly, all the gram-negative strains reported in the present study were found to be ESBL positive. The outbreak of multidrug-resistant and extended-spectrum β-lactamase-producing P. aeruginosa, C. davise, A. baumannii and Proteus mirabilis has been reported from hospital environment of Italy [93], Ethiopia [94], Korea [95], Malaysia [96], Mexico [97] and United States [98] and India [89]. Various healthcare-associated infections primarily by Achromobacter xylosoxidans has been reported in hospital settings of China [92], Tanzania [91], French West Indies [99].
Synergism and antagonism among indigenous bacteria is a common phenomenon. The present study reports antagonistic activity by the S. maltophilia strain GCC_19W2, P. aeruginosa strain GCC_19W3 and Achromobacter sp. against the tested bacterial strains. A similar study was conducted by Afrin, Bhuiyan [100] demonstrated the antagonistic activity of Bacillus amyloliquefaciens against multidrug-resistant Serratia rubidaea. Another study reports growth inhibition of Micrococcus luteus and Staphylococcus aureus by antibiotic-producing Bacillus and Lysobacter [101]. The bactericidal action of the isolated strains suppresses the growth of other tested microorganisms, possibly through competition for nutrients or secreting the inhibitory substances [102]. Microorganisms may also indirectly inhibit or reduce the growth of other microorganisms by changing pH, osmotic pressure and surface tension [100]. Detailed knowledge of synergism and antagonism among the pathogenic and non-pathogenic group of indigenous bacterial isolates is needed to prevent the emergence of drug-resistant strains, chronic toxicity, as well as the potential loss of normal microbiota upon interaction.
CONCLUSION
The present study demonstrates the outrage of different pathogenic bacteria isolated from nosocomial environments. All the isolates showed significant growth at pH 6–8 and are able to survive at 45 °C. P. aeruginosa strain GCC_19W3, Bacillus sp. strain GCC_19S2 and A. spanius strain GCC_SB1 exhibited β-hemolysis, which is an indicator of the virulence factor. A moderate potency of biofilm formation and colonisation of bacteria in the host cells was confirmed by auto-aggregation and cell-surface hydrophobicity analysis. These conducive results ascertain their adhesion to biotic and abiotic surfaces and may result in disease transmission. It has been observed that prolonged exposure of isolates to clinical effluent and toxic chemical might lead to the co-selection of antibiotic resistance and multi-metal tolerance. The gram-negative strains were able to produce β-lactamase enzyme which may pose serious public health risks. Antagonistic activity against other bacteria was evidenced by S. maltophilia strain GCC_19W2, P. aeruginosa strain GCC_19W3, A. spanius strain GCC_SB1 and A. xylosoxidans strain GCC_SB2. In conclusion, the study suggests urgent awareness and measures control the spread of emerging pathogens within the hospital biome and community. Further studies are also needed to assess the transmission of disease and the mechanism of pathogenesis.
Acknowledgement
The authors gratefully acknowledge DBT, New Delhi for providing financial assistance to setup Institutional Biotech Hub and Bioinformatics Centre in Gurucharan College, Silchar, India.
Notes
Conflict of interest
The authors declare that they have no competing interests.
Funding
The study was not directly funded by any agency or organization; however, laboratory facilities were provided by the institution.
Ethical approval
The study was approved by the Institutional ethical research committee.
Informed consent
Not applicable
CRediT author statement
SN: Conceptualization, Methodology, Supervision, Writing-Reviewing and Editing; AS: Experimentation, Investigation, Visualization, Results compilation, Writing-original draft preparation; YSS: Experimentation, Investigation, Visualization, Results compilation, Writing-original draft preparation; AD: Experimentation, Investigation, Visualization, Results compilation, Writing-original draft preparation; NB: Experimentation, Investigation, Visualization, Results compilation, Writing-original draft preparation; BD: Project administration, Supervision