Risk Assessment of Lead and Cadmium in Drinking Water for School use in Nakhon Si Thammarat Province, Thailand
Article information
Abstract
This research aimed to evaluate children’s health risk based on the concentration of lead (Pb) and cadmium (Cd) in the drinking water used by 44 primary schools. Samples were collected from bottled water, tap water, filtered tap water and raw water, for a total of 146 samples, between 1 September 2018 and 31 January 2019. The concentrations of Pb and Cd in drinking water samples were determined by graphite furnace atomic absorption spectrometry. The results showed that the concentration of Pb and Cd in bottled water samples were in the range of non-detected (ND)–0.0180 mg/L and ND–0.0013 mg/L, respectively. The concentration of Pb and Cd in tap water samples ranged from ND-0.0250 mg/L and ND-0.0042 mg/L, respectively, from ND–0.005 mg/L and ND–0.0021 mg/L, respectively, in filtered tap water samples and from ND–0.0400 mg/L and ND–0.0049 mg/L, respectively, in raw water samples. The summation of the total hazardous index (HI)-values of bottled water samples, tap water samples, filtered tap water, and raw water samples were less than 1, was considered health-protective. The results will provide the direct evidence needed by school managers to warn learners about the health risk of ingestion exposure among children.
The promotion of school hygiene is typically covered along with topics like food borne disease, water borne disease, hand washing with soap, waste management and clean drinking water. WHO/UNICEF [1] presented data that 31%, 29%, and 79% of schools lack basic water facilities, basic sanitation facilities and basic hand washing facilities, respectively. There are various categories of drinking water, including improved sources, such as piped water and boreholes, and unimproved sources, which include surface waters and unprotected drinking wells [1]. Quality of drinking water in primary schools is well recognised in its importance because adequate water intake not only presents a quality of health in children, but it may also have a positive impact on cognition of children [2–4]. The heavy metals in drinking water most often linked to human poisoning are lead (Pb), cadmium (Cd) and arsenic (As), which can be toxic even in low doses [5]. These studies indicate the potential for significant exposure to heavy metals to occur as a result of heavy metal contamination of school drinking water sources.
Shotyk and Krachler [6] reported that water bottled in glass containers was contaminated with Pb due to leaching from the containers. Heavy metal concentrations are low when the water leaves the water supply, but the heavy metal concentrations may increase during transportation and storage in the distribution center [7]. Fertmann et al. [8] reported a concentration of Pb in tap water higher than the detection limit and showed significantly higher blood Pb levels. Badr et al. [9] found concentrations of chromium (Cr), Pb and iron (Fe) in drinking water that there were significantly higher in tap water samples compared to bottled water. In addition, blood creatinine and urea levels were positively correlated with Cr and Cd levels in subjects who were mainly drinking tap water. Filtration is a common way to obtain pure drinking water by removing particles and microorganisms based on size exclusion. Conventional water treatment systems were found, in general, while advanced technologies such as Actiflo® clarification system, ultra-membrane filtration, dissolved air flotation (DAF) and ozone were only used at a few water treatment plants [10]. Ultra-membrane filtration uses transmembrane pressure to remove Cr, Cd, zinc (Zn), copper (Cu), nickel (Ni), and Pb, with removal percentages ranging from 92% to 100% [11]. The neurotoxic effects of Pb in children, even at low doses, are well understood [12,13]. Cd is extremely toxic, even at low concentrations, bioaccumulates in organisms and ecosystems, and has a long biological half–life in the human body, ranging from 10 to 33 years. Long-term exposure to Cd also induces renal damage. The contamination of water is directly related to water pollution, and the quality of ground and surface water sources needs to be assessed [14].
The main sources of drinking water in the primary schools are bottled water, tap water and filtered water. The water supply from surface water is used as drinking water in a few schools and raw water is also used to clean the containers, clean vegetables, etc. The concentrations of both Pb and Cd and the health risk from exposure need to be considered. The aims of this study were to investigate the concentration of Pb and Cd in drinking water samples and to assess the health risk from exposure of Pb and Cd in drinking water, which is used to service schools.
Materials and Methods
This cross-sectional study was conducted between 1 September 2018 and 31 January 2019 in public primary schools located in four districts, including the Phipun, Chawang, Chang Khang and Tham Phannara districts in the Nakhon Si Thammarat province, Thailand, which is supported by Nakhon Si Thammarat primary education service area 2. 44 public primary schools were selected using a purposive sampling method. A total of 146 drinking water samples were collected, including 48 bottled water samples, 44 tap water samples, 30 filtered tap water samples and 24 raw water samples.
Samples collection, Samples preparation, Measuring, and Analysis
Drinking water samples were collected from sources of drinking water and in the primary schools including 48 bottled water, 44 tap water, 30 filtered water and 24 raw water. The drinking water samples were collected in prewashed. Most of the drinking water samples were obtained directly from tap after allowing the water to run for at least 5 minutes. Water samples were stored in 50 polyethylene bottles previously washed in nitric acid. The water samples were acidified with 50% (v/v) nitric acid (E. Merck, Darmstadt, Germany) to brink the pH to less than 2 and analyzed within two days. All samples were filtered prior to analysis using Whatman® Ashless Filters, Grade 541, (Whatman, London, UK) to reduce the suspended solids associated with the risk of obstructing the capillary tubing in the instrument.
Pb and Cd concentrations were determined by graphite furnace atomic absorption spectrometry (GFAAS, PerkinElmer, Analyst 800). The standards and dilution standards for analyses were prepared by using 1.0 mol/L of nitric acid to dilute stock solutions of Pb and Cd (1000 mg/L). The calibration solutions and 20 μL of samples were pipetted into the graphite tube for measurement. The standard reference material (Trace element dissolved in water) was used for evaluating methods used to measure Pb and Cd in the drinking water samples. All samples were analyzed in triplicate. Detection limits of GFAAS were 0.0020 mg/L for Pb and 0.0001 mg/L for Cd.
Risk assessment
The test method of the US Environmental Protection Agency (U.S. EPA 1989) [14] was used to calculate chronic daily intakes (CDI) from incidental ingestion of Pb and Cd in drinking water as follows:
where C is the concentration of Pb and Cd in drinking water (mg/L) (data from laboratory analysis), IR is the ingestion rate (2 L/day for age over 6 years old, U.S. EPA 1989) [15], EF is the exposure frequency (360 days/year) [16], ED is the exposure duration (6 years) [16], BW is the body weight (average of body weight of students 35 kg, from questionnaires), AT is the average time of exposure (ED×365 days/year) [17]. When hazardous quotients (HQ) and hazard index (HI) values are less than one, there is no risk to the population, but if these values exceed one, there may be concern for potential non-carcinogenic effects [15].
Data analysis
Statistical analyses were performed using the statistical package SPSS 15.0. Descriptive statistics were applied to determine concentrations of Pb and Cd in the drinking water from the public primary schools.
Results
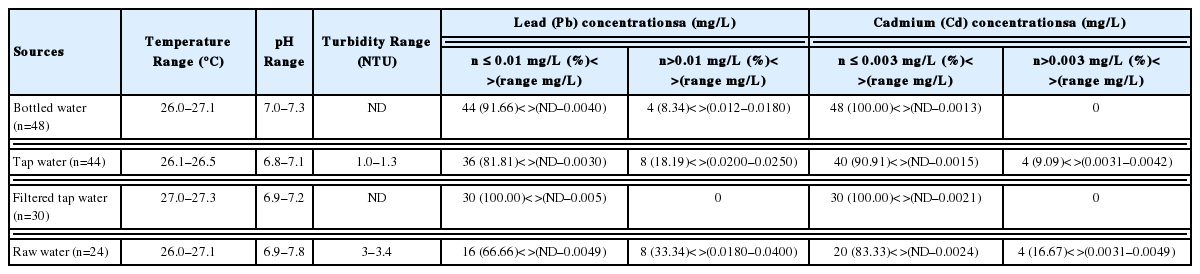
The samples of drinking water were collected for analysis from 44 primary schools. The following water quality parameters were measured: temperature, pH and turbidity. The temperature, pH and turbidity ranges of bottled water samples (48 samples) were 26.0–27.1°C, pH 7.0–7.3 and not detected (ND), respectively. For tap water samples (44 samples), the temperature, pH and turbidity ranges were 26.1–26.5°C, pH 6.8–7.1 and 1.0–1.3 NTU, respectively. The ranges for the filtered tap water samples (30 samples) were 27.0–27.3°C, pH 6.9–7.2 and not detected and the ranges for the raw water samples (24 samples) were 26.0–27.1°C, pH 6.9–7.8, and 3.0–3.4 NTU, respectively, for temperature, pH and turbidity.
The concentration of Pb and Cd ranged from ND–0.0180 mg/L and ND–0.0013 mg/L in bottled water samples, respectively, from ND–0.0250 mg/L and ND–0.0042 mg/L in tap water samples, respectively, from ND–0.005 mg/L and ND–0.0021 mg/L in filtered tap water samples, respectively, and from ND–0.0400 mg/L and ND–0.0049 mg/L in raw water samples, respectively (Table 1).
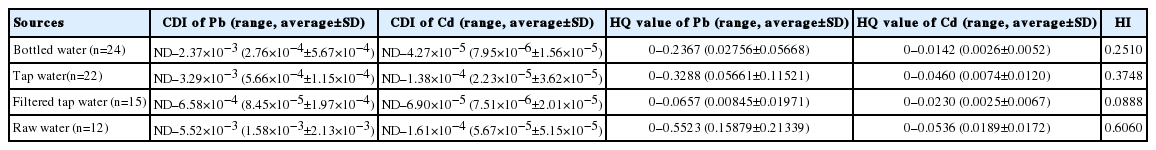
An estimation of CDI from the drinking water through ingestion showed that Cd intake was lower than Pb intake in all samples (Table 2). The highest Pb intake was found in raw water (5.52 × 10–3 mg/kg-d), while the greatest Cd intake was also found in raw water (1.61 × 10–4 mg/kg-d). With respect to HQ that are regularly applied to evaluate human health risk from intake of drinking water through the ingestion pathway, the greatest HQ of Pb and Cd was 0.5523 and 0.0536 in raw water, respectively. The summation of the total HI-values of bottled water samples, tap water samples, and raw water samples were 0.2510, 0.3748, 0.0888 and 0.606, respectively (Table 2).
Discussion
The results of temperature, pH, and turbidity of all samples in this study did not exceed the recommended permissible limits set by Thai ministry of public health and World Health Organization (WHO) [18, 19]. The concentration of Pb found in 8.34% of the bottled water samples exceeded the recommended permissible limit of Pb in guidelines for drinking water quality. In contrast, the Cd concentration in bottled water samples did not exceed the permissible value of Cd for the drinking water quality standard. Bottled water in Thailand comes from many different brands and types, primarily based on the purification process, including reverse osmosis, ultraviolet treatment, ozone treatment and mineral or spring water. However, a portion of bottled water brands in this study were produced in a factory from the local community, and the production process has yet to be standardised, as Pb was detected in some of the brands. However, the large bottled water companies in Thailand have large water purification facilities and are more likely to stick to a good standardized production process.
The concentrations of Pb and Cd in tap water samples were 18.19% and 9.09%, respectively, which exceeded the permissible values in guidelines for drinking water quality. Some studies reported the dissolution of Pb in tap water when Pb free taps were used, which was attributed to in-line brass fittings within household plumbing systems, thus, suggesting the use of a tap made from a material that does not contain Pb, such as stainless-steel taps [20, 21]. In addition, flushing the taps before consumption for two or three minutes is a technique that can be help reduce the concentration of heavy metal contaminants [20, 22, 23]. Furthermore, some suggest that the presence of chlorine and other pollutants in tap water, such as trihalomethanes (THMs), potentially create a commonly formed carcinogen, such as chloroform [24]. In addition, fluoride can be present in tap water, particularly in the mountains, as there is naturally more fluoride in the water there than elsewhere. In Thailand, the highest fluoride content was found in samples from the central and eastern regions [25]. An excess of fluoride in the water can have detrimental effects on teeth, especially during the phase of growth. Some studies clearly established that fluoride primarily produces effects on skeletal tissues (bones and teeth) [26,27].
The concentration of Pb and Cd in filtered tap water samples did not exceed the permissible value in guidelines for drinking water quality set by Thai ministry of public health and WHO [18, 19]. Khulbe and Matsuura [28] reported that membranes for membrane adsorption, which have the dual function of membrane filtration and adsorption, are very effective in removing trace amounts of pollutants, such as cationic heavy metals, anionic phosphates and nitrates. Al-Rashdi et al. [29], reported the removal of some heavy metals including Cu, Cd, manganese (Mn), Pb and As from aqueous solutions using adsorption and nanofiltration membrane technologies.
The concentration of Pb and Cd in 33.34% and 16.67% of raw water samples, respectively, exceeded the recommended permissible limit in guidelines for drinking water quality. The quality of surface water in an area is highly affected by human activities in that area [30]. In this study, agricultural activities may have a profound effect on the surface water [31,32] because the agricultural areas, para rubber, rice farming and garden fruit, were located near the surface water source. Agricultural activities can be related to the surface contamination of pesticides, heavy meals and other pollutants. Thus, raw water samples are not suitable for drinking purposes unless it is treated by the water agencies.
The summation of the total HI-values of bottled water samples, tap water samples, drinking water samples and raw water samples were less than 1; suggesting there was considered health-protective. However, minimizing potential human exposure is still adverse health effects. Based on several studies, Pb is best known for impeding children’s growth, as it accumulates in the long bones and causes bone damage [33–36]. In addition, low Cd exposure is still limited and has had opposing results. Data reported from the National Health and Nutrition Examination Survey (NHANES) in U.S. showed a positive link between child urinary Cd and the prevalence of learning disabilities and special education among 6–15-year-olds, however, a non-significant lower prevalence of attention deficit hyperactivity disorder (ADHD) was related to the exposure [5].
Conclusion
Pb and Cd concentrations were determined from the drinking water of select primary schools in Nakhon Si Thammarat province, Thailand. The concentrations of Pb and Cd detected in filtered tap water did not exceed the recommended permissible limits. The results provide the direct evidence needed by school managers to warn learners about the health risk of ingestion exposure among children. Thus, should control the production of bottled water in local water production factories to ensure the factories have the appropriate technology to meet the drinking water standards. In addition, raw water is not suitable for drinking purposes unless it is treated by the water agencies. However, toxicity tests such as in vitro or in vivo toxicity test, are important components in assessing the impact of heavy metals on aquatic ecosystems because they reveal toxic effect of complex chemical mixtures [37]. Thus, toxicity tests should be carried out in the drinking water.
Acknowledgement
This research was supported by the Faculty of Health and Sports Science, Thaksin University.
Notes
Conflict of interest statement
The author has no conflict of interest associated with the material presented in this paper.