Experimental modeling of the acute toxicity and cytogenotoxic fate of composite mixtures of chromate, copper and arsenate oxides associated with CCA preservative using Clarias gariepinus (Burchell 1822)
Article information
Abstract
Concurrent occurrence of chromium (Cr), copper (Cu) and arsenic (As) from chromated copper arsenate (CCA) wood preservative in aquatic ecosystems demands that their joint-actions in eliciting toxic effects be assessed for adequate understanding of the health risk they may pose to biota. Clarias gariepinus was exposed to As2O3 , CrO3 and CuO and their composite mixtures (1:1 and 1:1:1) at various concentrations (0 – 600 mg/L) for 96-h to determine the acute toxicity using OECD (1992) protocol. C. gariepinus was then exposed to sub-lethal concentrations corresponding to 6.25, 12.5, 25.0, 50.0 and 100% of the 96-h LC50 for 7 days to assess the cytogenotoxic effects using piscine micronucleus (MN) test. The 96-h LC50 showed that the metals/metalloid demonstrated differential interactions in a concentration dependent pattern. The 96-h LC50 showed that Cr was the most toxic while Cu and As:Cu were indeterminate (Cr > Cr:Cu > As:Cr > As > As:Cr:Cu > Cu = As:Cu indeterminate). Isobologram and synergistic ratio (SR) models predicted antagonistic interaction between Cu:Cr and As:Cr and synergism between As:Cu in the causation of morbidity and mortality of C. gariepinus. Interaction factor model predicted antagonism as common interactive mechanism among the metal/metalloid mixtures in the induction of MN and abnormal nuclear erythrocytes in C. gariepinus. Predicted interactions among the three metals/ metalloid were largely antagonism and synergism towards the induction of acute toxicity and cytogenotoxicity. The models employed herein may be useful in establishing environmental safe limits for mixtures of metals/metalloids against the induction of acute toxicity and DNA damage in lower aquatic vertebrates.
Introduction
Accelerated urbanization and industrial developments are the major cause of unprecedented increase in worldwide chemical discharge into the environment. In most developing nations, generated chemicals are poorly managed by habitual illegal disposal on available spaces in the environment. Moreover, poor enforcement of laws and regulations prohibiting improper discharge of hazardous substances further encourages culprits. Most of the discharged chemical and physical agents cause great alterations in the biogeochemical circles of both aquatic and terrestrial ecosystems and in turn compromising human and ecosystem health [1]. Metals are the most commonly released chemicals into the environment via multiple direct and indirect sources [2]. A seemingly neglected direct source of toxic metal discharge is through chromated copper arsenate (CCA) wood treatment preservative [3-8]. The individual metals in CCA preservatives exist in varying compositions, hence they are classified into Types A, B and C. The metal compositions range from 2100 - 2300 mg/L chromium trioxide (CrO3), 1200 - 1400 mg/L copper oxide (CuO) and 1800 - 2200 mg/L arsenic trioxide (As2O3). This is equivalent to 65.5% (Cr), 18.1% (As) and 16.4% (Cu) in Type A, 35.3% (Cr), 19.6% (As) and 45.1% (Cu) in Type B, and 47.5% (Cr), 18.5% (As) and 34.0% (Cu) in Type C [9]. Irrespective of the variations in the percentage compositions of individual elements in CCA, the leachability of the metals into the aquatic and terrestrial environments, is unaffected [5-7, 9-10]. Furthermore, studies have shown that individual metals in CCA readily leach from treated woods and timbers into aquatic and terrestrial environment at varying proportions and persist for about four decades [4,7, 10-15]. Considering that CCA has a worldwide acceptance due to its effectiveness in protecting woods and timbers from fungal and insect attacks and its extensive usage for decades [9], may suggest threat to biota due to the high concentrations of the component metals/metalloid in the environment.
The individual metals/metalloid in CCA: chromium (Cr), copper (Cu) and arsenic (As), are clastogens and or aneugens, teratogens, endocrine disruptors and potential tumor inducers [16-18]. Due to increasing health risk and environmental contamination ascribed to these metals, in 2003 all arsenic-based wood preservatives including CCA were restricted from used as preservative for decks and patios, picnic tables, playground equipment, walkways/boardwalks, landscaping timbers, and/ or fencing by the U.S. Environmental Protection Agency [19]. Despite its restriction, existing CCA-treated woods and structures will persistently leach Cr, Cu and As into the environment for periods between 10 - 40 years [7,11,15]. This suggests that Cr, Cu and As will continue to contaminate aquatic and terrestrial environments via wood wastes from construction, demolition and remodeling projects. Moreover, in many developing countries, CCA preservative is still in use either deliberately or illegally due to its effectiveness in protecting timbers and utility poles [20]. CCA coated timbers and poles are sometimes submerged in large water bodies to prolong their life shelves. This act leads to increase in leaching and direct release of Cr, Cu and As into the water bodies [21]. In a recent study, Sogbamu et al. [22] asserted that the degradation of aquatic environment by illegal submerging of treated woods and timbers in Lagos Lagoon, is a common practice in Lagos State, Nigeria. It becomes imperative to routinely monitor these metals and assess their deleterious impacts in most environmental media so as to protect biota and human health. Cr, Cu and As leaching from CCA persist in the aquatic sediments and water, soils and readily bio-accumulate in biota, particularly epibiotic organisms, that live on treated woods [23]. This poses a deleterious effect on animal diversity and subsequently human health [4]. For instance, American oysters (Crassostrea virginica) living in a CCA contaminated water body, bio-accumulated up to 600 mg of Cu which in turn significantly induced pathological lesions and clastogenicity in the Oysters’ tissue [24,25]. Aquatic environment is particularly susceptible to the accumulation of Cr, Cu and As during leachability from CCA treated wood, howbeit, there is dearth of information on the potential joint action toxicity of the metals in eliciting morbidity and mortality, growth retardation and induction of genetic instability in aquatic vertebrates. An increasing area of research focus in recent times, is the assessment of interactive effects among individual metals; which may lead to eliciting greater or lesser toxicity in biological systems via synergistic, antagonistic and/or additive interactive mechanisms [26-28]. The report herein is the first to utilize multi-model approach and cytogenetic biomarker to investigate the possible joint action toxicity of oxides of Cr, Cu and As on aquatic vertebrates using Clarias gariepinus as a model organism.
Materials and methods
Chemicals
As2O3 (CAS No. 1327-53-3), CrO3 (CAS No. 1308-38-9), CuO (CAS No. 1317-38-0) and benzene in analytical grades were obtained from Sigma (St. Louis, MO).
Animals
Juvenile C. gariepinus (mean ± SD body weight 11.00 ± 1.00 g and length 9.40 ± 1.50 cm) obtained from the Fisheries Department, Oyo State Ministry of Agriculture and Natural Resources, (Ibadan, Nigeria) were used for this study. They were acclimatized for 14 days to laboratory conditions of 26.0 ± 1.0°C and 12 h dark/light cycle prior to the experimental set-up. They were stocked at a population density of 10 fish per 25 L transparent aquarium containing underground borehole water and fed twice daily with standard fish meal manufactured by Coppens®.
Study design
Single action toxicity and joint action toxicity assessment of the metal solutions
Ten juvenile C. gariepinus per group, randomly selected into seven experimental groups per individual metal concentrations and their composite mixtures, were utilized for the 96-h acute toxicity testing in accordance with standard regulations and guidelines for testing of chemicals [29]. Following range finding tests analysis for the metal oxides; 0 - 600 mg/L of the individual metals and their composite mixtures were selected for the acute toxicity study. The metal oxide solutions were prepared immediately before use and the C. gariepinus were not fed during the acute toxicity study (static bioassay). Mortality and clinical signs of toxicity were recorded by visual examination at every 24 h during the 96-h exposure duration. Fish were considered dead when no movement was observed after gentle prodding with a glass rod. Environmental relevant safe concentrations (SC) for the individual metals/metalloid and their composite mixtures; As:Cr, As:Cu, Cr:Cu and As:Cr:Cu (composite mixture as used herein referred to equal molar volumes of the metals corresponding to the selected percentages of the LC50 of individual metals in binary ratio=1:1 and tri-metal ratio=1:1:1), at 96-h exposure were derived by multiplying the 96-h LC50 by a factor of 0.1 in accordance with European Inland Fisheries Advisory Commission (EIFAC) [30].
Sub-lethal toxicity and piscine micronucleus analysis
Five concentrations corresponding to 6.25, 12.5, 25, 50 and 100% of the 96-h LC50 in mg/L (metal solution/underground borehole water) for each of As, Cr, As:Cr, Cr:Cu and As:Cr:Cu were selected for the sub-lethal toxicity study (Table 1). Similarly, 6.25, 12.5, 25, 50 and 100% of 100 mg/L (metal solution/ underground borehole water) each for Cu and As:Cu were selected in accordance with standard guidelines for testing of chemicals [29], since the 96-h LC50 of Cu and As:Cu, were indeterminate. The metal solutions were prepared immediately before use and each experimental set-up was renewed every 48-h to ensure constant exposure of test organisms to the metal oxide solutions and also avoid accumulation of metabolic wastes and remains of food particles. Similar treatment was given to fish exposed to borehole water as negative control and 0.10 mL/L of benzene, a hematopoietic genotoxic inducer [31], as positive control. Fish in the control and exposed groups were weighed (initial weight gain) before the commencement of exposure using Acculab® USA, Model-vic-303 electronic analytical weighing balance. At 7-day post exposure, fish from the control and exposed groups were weighed (terminal weight gain) and peripheral blood collected through the caudal vein for slide preparation. Thin blood smears were prepared on three pre-clean and grease free microscope slides per fish [32]. The prepared slides were allowed to air dry for 24-h, fixed in absolute (98%, v/v) cold methanol (4 °C) for 30 min and counter stained with 5% Giemsa and May-Grunwald stains for 20 min. 2000 cells per slide were analyzed for MN and nuclear abnormalities (NAs) in accordance with standard protocols [33,34].
Statistical analysis
Analysis of the concentration - response data
Data obtained from the 96-h mortality were analyzed using probit method with SPSS 16.0® computer program. The analyzed acute toxicity indices were presented as LC5 (sub-lethal concentration that induced 5% mortality of test model), LC50 (median lethal concentration that induced 50% mortality of test model) and LC95 (lethal concentration that induced 95% mortality of test model). Toxicity factor (TF) for 24 hourly relative potency measurements for the metals were determined in accordance with standard procedure [28]:
The interactive effects of individual metals in the composite mixtures were defined by the following models:
Synergistic ratio (SR) model: This model predicts the metal that greatly contributed to the observed toxicity in metal mixture [35]. It is determined as follows:
For this interaction model: when SR=1 it is additive; SR < 1 it is antagonistic; and SR>1 it is synergistic interactions.
Concentration–addition model: This model assumes that the addition of similarly acting toxicants are mixed in varying proportions, they will add up to give the observed response [36]. In evaluating the joint-action, a predicted LC50 is derived by summing up the LC50 values of the individual toxicants according to the proportion of their contribution in the mixture. The predicted LC50 is then compared with the observed LC50 of the mixture. The outcome classifies the pattern of interaction among the component metals of the mixture. The relationship between the derived LC50 and predicted LC50 (RTU) is estimated as:
For this interaction model: RTU=1 explains addictive; RTU < 1 explains antagonistic and RTU>1 explains synergistic interactions.
Isobologram describes the graphical presentation of jointaction toxicity of individual metals in a binary mixture [37]. Isobologram was used to show the pattern of interactions between individual metals in the binary mixture of the metal solutions that produced the biological response during the 96-h acute toxicity (96-h LC50).
The percentage weight gain of fish was determined using the formula:
Micronucleus test analysis
The data obtained for MN and NAs are presented as mean ± standard error (SE). One-way analysis of variance (ANOVA) was used to determine the difference (p=0.05) among the various exposed and control groups. Significant difference between each treatment group and the negative control was determined using Dunnett multiple post-hoc test (DMPT) at p=0.05. The interaction factor (IF) between the binary and among the tri-metal constituents that led to the induction of MN and NAs, were determined in accordance with Katsifis et al. [38] and Danesi et al. [39] formula with slight modification:
where CCA is the statistical mean of the assessed biomarker induced by the mixtures of CCA solutions; C, C and A are means of biomarkers induced by individual metals present in the binary and tri-metal mixtures; while T is the mean of the negative control (borehole water).
Also, standard error (SEIF) for IF calculation is derived from:
where SEIF is the standard error of IF; SECCA, SEC, SEC, SEA, and SET are standard error for CCA, C, C, A and T respectively. When IF value is negative, it is antagonism; when it is positive, it is synergism; and when it is zero, it is additivity.
Results
Joint-action toxicity of binary and tri-metal mixtures of chromium, copper and arsenic on Clarias gariepinus
The individual metal solution and their mixtures induced different rate of mortality on juvenile stage of C. gariepinus. Cu alone and As:Cu mixture did not induce any mortality even at the highest selected concentrations (600 mg/L). Mortality was only recorded at higher concentrations (300 - 600 mg/L) for As and As:Cr:Cu mixture, while mortality increased with concentrations for Cr, As:Cr and Cr:Cu. 100% mortality of the test model was recorded at 150 mg/L Cr, 300 mg/L As, As:Cr and Cr:Cu and 450 mg/L As:Cr:Cu (Figure 1). The 24-h LC5, LC50 and LC95 revealed that the concentration dependent increase mortality induced by Cr>Cr:Cu>As:Cr>As>As:Cr:Cu in that order, was also exposure duration related. This shows that Cr (96- h LC50=61.68 mg/L) was the most toxic metal (Table 2) and Cu and As:Cu the least toxic since there was no observed mortality at all tested concentrations (96-h LC50=indeterminate). The environmental SC for Cr, As, As:Cr, Cr:Cu and As:Cr-Cu with respect to the juvenile sized C. gariepinus used herein are 6.17, 21.30, 12.28, 10.04 and 25.46 mg/L respectively. Also, the derived TF showed that Cr was 4.13 fold more toxic than As:Cr:Cu (TF=1.00) (Table 2).

Percentage mortality of juvenile Clarias gariepinus exposed to varying concentrations of individual and composite mixtures of chromium (Cr), copper(Cu) and arsenic (As) oxides for 96 h. Number of fish per concentrations=10 fish.

96-h acute toxicity of individual and composite mixtures of chromium (Cr), copper (Cu) and arsenic (As) using juvenile stage of Clarias gariepinus
Possible mode of interactions among chromium, copper and arsenic solutions predicted from the mortality data obtained from exposed juvenile Clarias gariepinus
The experimentally determined 96-h LC50 values for Cr, Cu and As and their composite mixtures, suggest that some forms of interactions existed among the binary and tri-metal mixtures that led to the mortality of C. gariepinus. For instance, the composite mixture of Cr:Cu was less toxic to C. gariepinus compared to Cr (Table 2), but more toxic to C. gariepinus compared to Cu (96-h LC50=indeterminate). Similarly, the composite mixture of Cr:As was less toxic to the fish compared to individual Cr and As (Table 2). Meanwhile, the composite mixture of As:Cu did not induce mortality even at 600 mg/L similar response to Cu, but As was more toxic than As:Cu mixture to the fish. The tri-metal mixture; Cr:As:Cu (1:1:1) was less toxic to the fish compared to mortality responses from Cr and As, but more toxic than Cu.
The joint-action toxicity models present different predictions to explain the possible interactions involved. SR model predicted antagonism between As and Cr (0.290), synergism between As and Cu (2.130) and synergism between Cr and Cu (0.616) (Table 3). Similarly, the LC50 data determined for both individual and composite binary mixtures were fitted into Isobolograms and compared with the theoretical model derived by Tallarida [37] (Figure 2). The analysis predicted antagonism as possible mechanisms of interaction between As and Cr, and between Cr and Cu but synergism between As and Cu (Figure 2). While concentration-addition model predicted synergistic mode of interaction for all the composite binary mixtures; As:Cr (2.24), Cu:Cr (1.61), As:Cu (3.13) and tri-metal mixtures; As:Cr:Cu (1.47) (Table 3).

Synergistic ratio (SR) and concentration-addition unit (RTU) models derived from using the experimentally 96-h LC50 obtained for both binary mixtures and individual chromium (Cr), copper (Cu) and arsenic (As) oxides
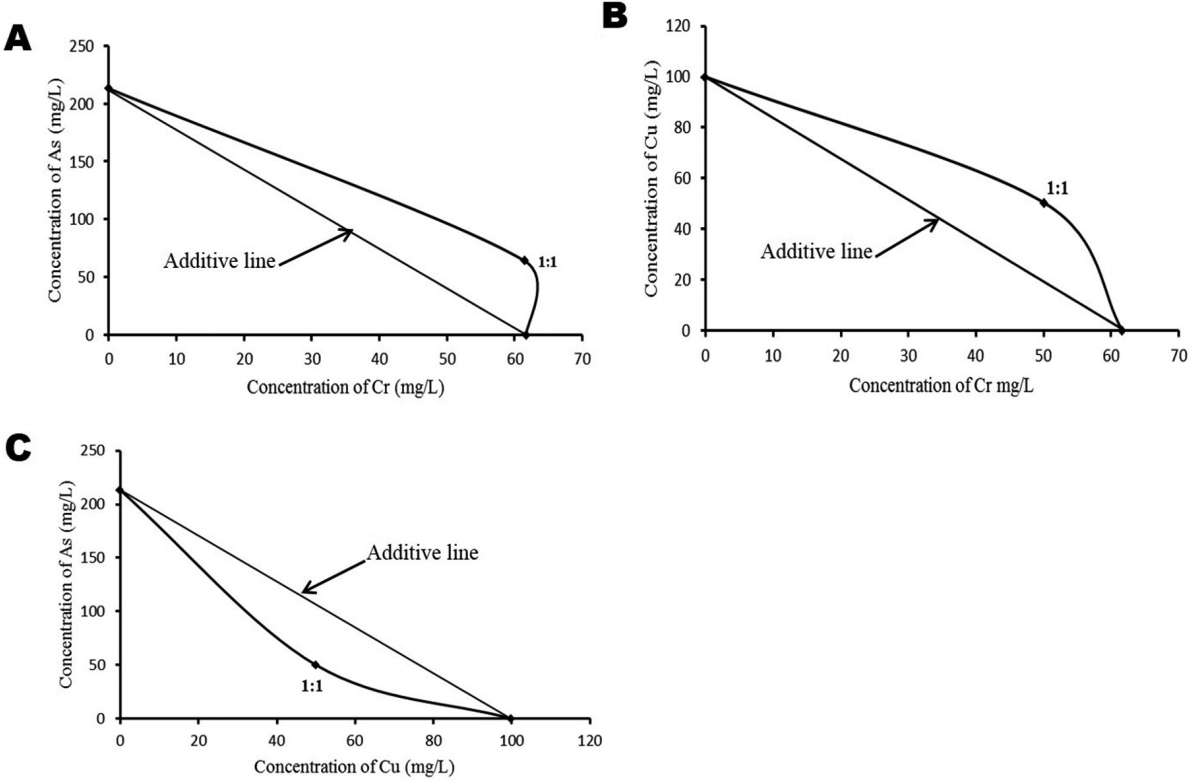
Isobologram presentations of the joint-action toxicity of the individual metals/metalloid using the experimentally obtained 96 h LC50 value for each metal/metalloid; Isobole (A) for arsenic(As) : Chromium (Cr) interaction depicts antagonism, Isobole (B) for copper (Cu): Cr interaction depicts antagonism while Isobole (C) for As:Cu interaction depicts synergism.
Clinical signs of toxicity and mortality induced by individual and composite mixtures in Juvenile Clarias gariepinus
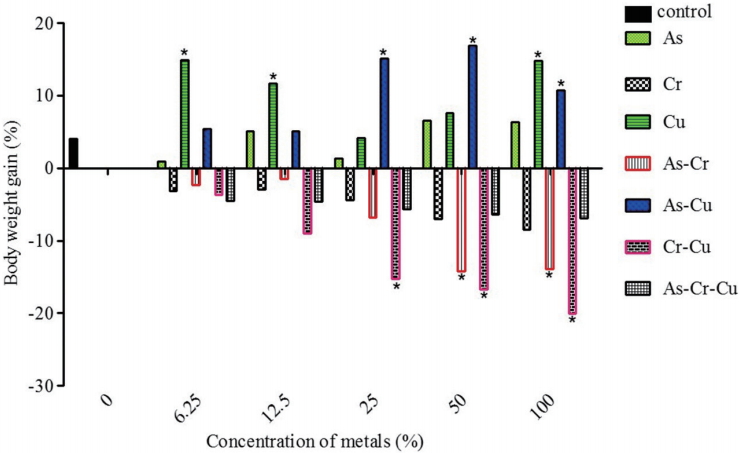
During the 96-h acute toxicity testing, the exposed fish showed various signs of toxicity in response to individual and composite mixtures of Cr, Cu and As exposure. Fish exhibited erratic and fast movements when newly introduced into metal solutions, with an attempt to jumping out of the aquarium. With exposure time they began to move sluggishly while becoming weak (signs of reduced activities) and eventually died. However, it is interesting to note that such erratic movement though common with As, but fish mortality occurred only at higher concentrations, as fish became adjusted and survived at lower concentrations of As. These signs were mostly observed in the following metal solutions and in the order: As>Cr>AsCr>Cr-Cu>Cr-Cu-As. Fish exposed to Cu and mixture of As-Cu did not display prominently such behavioral signs and they all survived even after the 96-h acute toxicity period at all tested concentrations. As, Cr and As:Cr induced some gross pathology. For instance, fish exposed to composite mixture of As:Cr presented ulceration of the genital opening. There were inflammation of the blood vessels at the ventral surface of fish exposed to As, skin lesion at the ventral view of a fish exposed to Cr solution, and ulceration of the genital opening of fish exposed to composite mixtures of As:Cr (Figure 3). During exposure to the selected sub-lethal concentrations (Table 1) of individual and mixtures of the metals/metalloid for 7 days, all fish in the treatment groups; Cr, Cr:Cu, As:Cr and As:Cr:Cu presented significant (p < 0.05) body weight loss compared to the negative control. While fish exposed to As, Cu and As:Cu metal solutions showed significant (p < 0.05) increase in body weight gain compared to negative control (Figure 4).
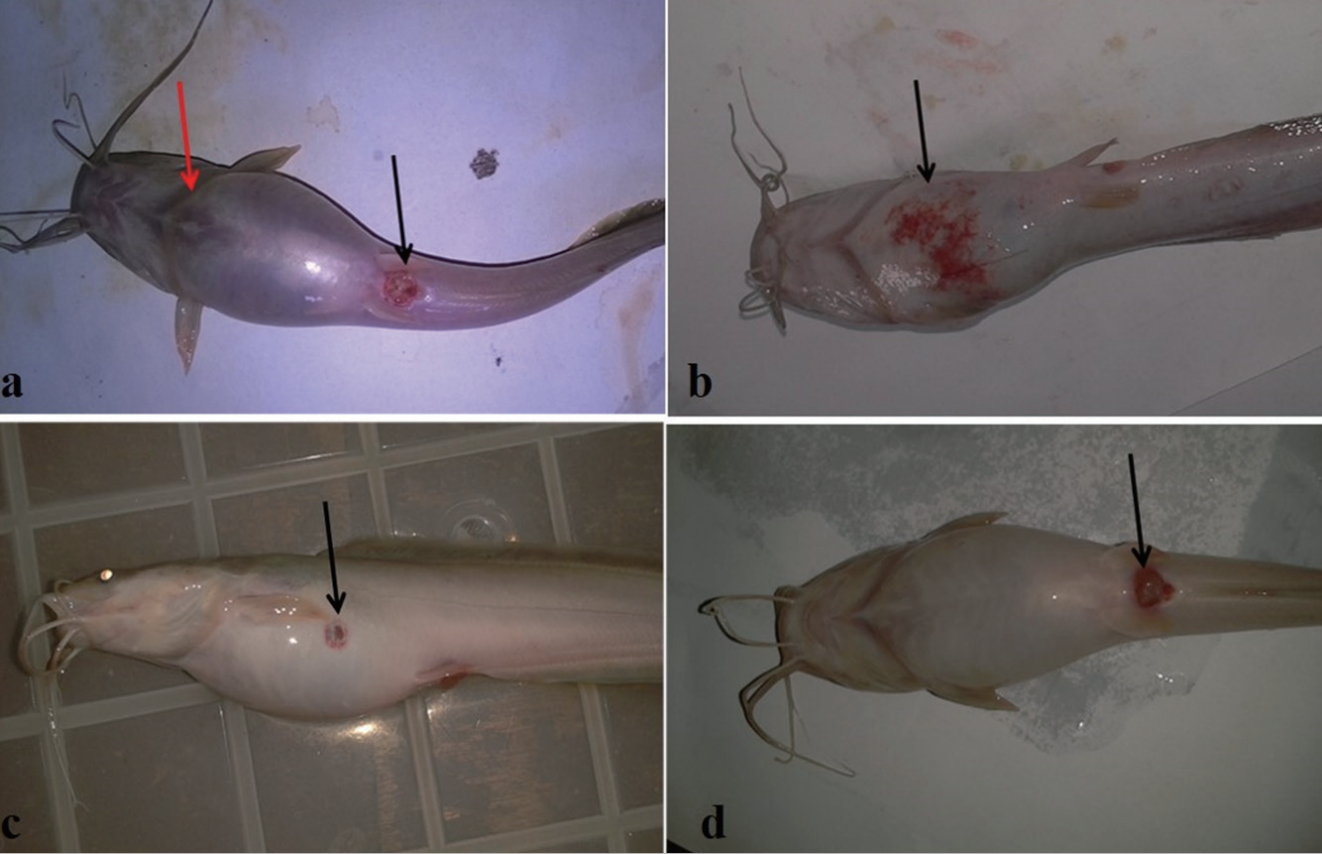
Clinical signs of toxicity observed in Clarias gariepinus exposed to varying concentrations of chromium (Cr), copper (Cu) and arsenic (As) oxides and their composite mixture for 96 h. (a) Swelling of the skin (red arrow) and ulceration of the genital opening (black arrow) in fish exposed to composite mixture of As:Cr solutions. (b) Inflammation of the vascular blood vessels at the ventral view (black arrow) of fish exposed to As solution. (c) Skin lesion at the ventral view (black arrow) of fish exposed to Cr solution. (d) Ulceration of the genital opening (black arrow) of fish exposed to composite mixtures of As-Cr solution.
Chromium, copper and arsenic and their composite mixtures increased frequencies of micronucleated erythrocyte and erythrocyte with nuclear abnormalities in peripheral blood of Clarias gariepinus
There was significant increase in the frequency of MN (Figure 5a) in peripheral erythrocytes of C. gariepinus treated with the various sub-lethal concentrations of individual and mixtures of metals/metalloid compared to the negative control. MN induction showed concentration dependent and positive correlation with metals; Cr (p < 0.0001, r=0.859, F=28.46), As:Cu (p < 0.0001, r=0.757, F=14.53), As (p < 0.0001, r=0.821, F=21.53), As:Cr:Cu (p < 0.0001, r=0.750, F=14.06); As:Cr (p < 0.0001, r=0.745, F=13.57), Cr:Cu (p=0.0004, r=0.558, F=5.90) and Cu (p < 0.0001, r=0.725, F=12.37) (Figure 6). At the 100% concentration which corresponds to 96-h LC50 values for the individual and mixtures of metals/metalloid, fold increase in frequencies of MN was in the order: Cr (4.33 fold)>As:Cu (3.83 fold)>As (3.78 fold)>As:Cr:Cu (3.50 fold)>As:Cr (3.33 fold)>Cr:Cu (3.22 fold)>Cu (2.83 fold), compared with the negative control. This indicates that Cr induced the highest frequency of MN while Cu induced the least.
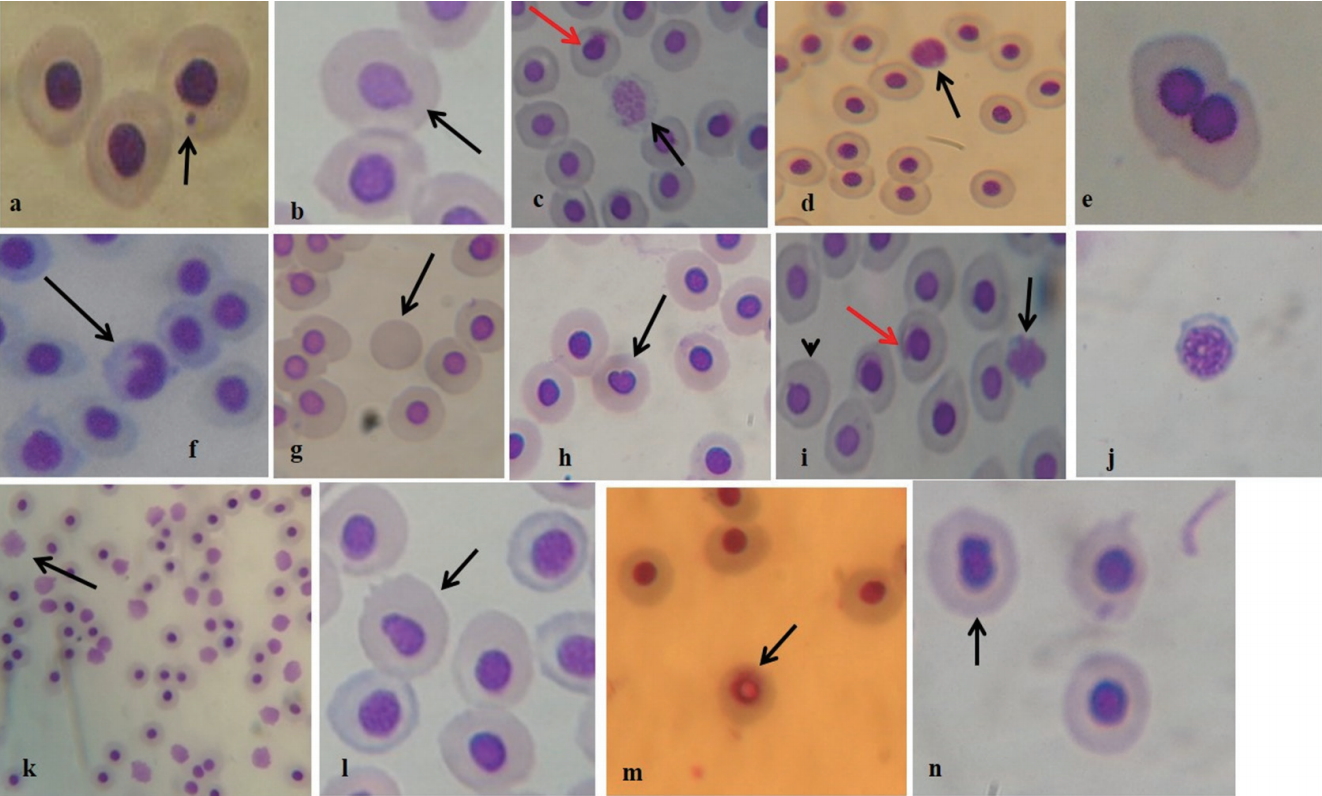
Micronucleated (MN) and abnormal nuclei erythrocyte formed in fish exposed to different concentrations of individual and mixture of chromium (Cr), copper (Cu) and arsenic (As) oxides for 7 days. (a) MN erythrocyte; (b) erythrocyte with nuclear bud; (c) nuclear fragmented erythrocyte (black arrow) and eroded nucleus (red arrow); (d) necrotic erythrocyte (e) binucleated erythrocyte; (f) erythrocyte with nuclear notch (g) enucleated erythrocyte; (h) erythrocyte with bleb nucleus; (i) early stage necrotic erythrocyte (black arrow), erythrocyte with nuclear bud (arrow head), eroding nucleus (red arrow); (j) early stage of nuclear fragmentation, (k) numerous necrotic erythrocytes (arrow), (l) erythrocyte with beans shaped nucleus (arrow), (m) erythrocyte with vacuolated nucleus, (n) erythrocyte with bilobe nucleus.

Frequency of micronucleated erythrocytes in peripheral blood of C. gariepinus exposed to arsenic (As), copper (Cr), copper (Cu), As:Cr, As:Cr, Cr:Cu and As:Cr:Cu. *p < 0.05; **p < 0.01; ***p < 0.001 are significantly different from the negative control using Dunnett’s multiple post hoc comparison test. Ben-Benzene (0.10 mL/L), NC- Negative control.
The metals similarly elicited concentration dependent significant (p>0.05) increase in total nuclear abnormalities (TNAs) in peripheral erythrocytes of treated C. gariepinus (Table 4). The frequently observed NAs; nuclear bud (Bud; Figure 5b), fragmented nucleus (FRA; Figure 5c), necrosis (NEC; Figure 5d) and binucleated erythrocytes (BN; Figure 5e) were scored individually and also as component of TNAs along with others not frequently observed NAs (Figure 5f-h). These include notch nucleus (Figure 5f), enucleated erythrocyte (Figure 5g), and bleb nucleated erythrocytes (Figure 5h). At 100% concentrations for individual and mixtures of metals/metalloid, the fold increase in frequencies of NAs was in the order: Cr (17.67 fold)>Cu (16.33 fold)>As:Cr:Cu (16.11 fold)>As (14.67 fold)>As:Cr (13.78 fold)>As:Cu (12.56 fold)>Cr:Cu (10.67 fold) when compared with the negative control. Cr induced the highest frequency of NAs, while As:Cu induced the least numbers of NAs. Surprising observation was higher necrotic and apoptotic erythrocytes that characterized erythrocytes from Cu treated fish (Figure 5k; Table 4).

Nuclear abnormalities (NAs) and total nuclear abnormalities (TNAs) in peripheral erythrocytes of Clarias gariepinus exposed to individual and composite mixtures of chromium (III) oxide (CrO3), copper oxide (CuO) and arsenate (III) oxide (As2O3)
Analysis of interaction factor
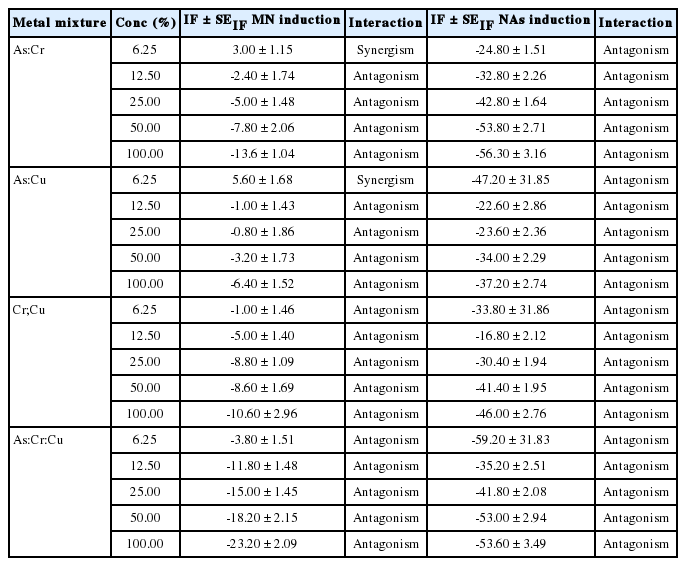
Analysis of the IF suggests antagonism as the common interactive mechanisms involved between the composite mixture of the tested metal solution in the induction of MN and NAs (Table 5) except for 6.25% concentration where As:Cu and As:Cr synergistically interacted to induce MN erythrocytes.
Discussion
Mixture of metals are commonly encountered in both aquatic and terrestrial environments and this suggests that biological species including humans are simultaneously and or concurrently exposed to myriad of these metals. CCA, a water-soluble wood preservative, has been shown to release Cr, As and Cu into aquatic environment via multiple routes. This includes leachates generated from landfills containing CCA‐treated wood [5,7], As, Cr and Cu leaching from CCA coated woods in household and public furniture, wood constructed bridges and enclosures [4,15,40] and most dangerous and direct route is via sub-merging CCA treated logs and timbers into water bodies [11]. This practice was implicated with decline in C. gariepinus population inhabiting Lagos Lagoon [23]. However, the mechanism(s) involved in the decline is largely unknown. Herein, acute toxicity and genotoxicity were utilized to unravel possible etiology associated with exposure to individual and mixtures of As, Cr and Cu in aquatic environment using C. gariepinus.
Variations in 96-h LC50 of 151.95 mg/L (Cr) for Mosquito fish (Gambusia affinis) [41], 119.52 mg/L for Tilapia nilotica, 87.93 mg/L for Cyprinus carpio, 53.57 mg/L for Ctenopharyngodon idella [42] and indeterminate LC50 for Brazilian fish, Piaractus mesopotamicus [43] compared to 61.68 mg/L for C. gariepinus in the study herein showed the influence of model organisms (species, developmental stages and health status) [44] in LC50 determination. Similarly, 96-h LC50 of 28.1 and 15.3 mg/L (As2O3) for zebrafish and rainbow trout respectively [45], 30.0, 24.5, 10.2 and 22.2 mg/L for Labeo rohita, Cirrhina mrigala, Catla catla and Ctenopharyngodon idella respectively [46] also showed variation from 213.0 mg/L As obtained for C. gariepinus in our study. Variations in LC50 values for the same metal in different model organisms suggest that the determination of LC50 for chemicals still remains an important step in preliminary toxicology and environmental risk assessment of chemicals [44,47]. That As and Cu in their binary mixtures with Cr reduced the mortality response of C. gariepinus compared to Cr treatment alone by 2.0 and 1.63 fold respectively, suggests As and Cu inhibited chromium absorption due to their potential direct interaction at the plasma membrane [48]. This assertion was supported by Isobologram and synergistic (SR) prediction models of antagonistic interactions between Cr:Cu and As:Cr. Studies are scarce that assessed joint-action interaction between Cu and Cr or As and Cr, however previous report on binary mixture (1:1) of Cu:Zn interaction was via antagonism in eliciting mortality response in juvenile Orechromis niloticus [27]. Similarly, varying the ratio of concentrations (4:1, 3:2 and 1:4) of binary mixture of Cu:Zn interacted antagonistically to elicit mortality response of T. fuscatus var radula [28]. Findings from our study suggest antagonistic interaction among individual metals in As:Cr:Cu and As:Cu in response to increase mortality of C. gariepinus. This finding may be useful in the management of CCA polluted sites and setting environmental safe limits for freshwater bodies.
Studies have shown that Cu has the highest accumulation potential (Cu>As>Cr) in sediments and benthos from CCA polluted sites [10,23,25]. For instance, American oysters (Crassostrea virginica; an epibiotic organism) inhabiting CCA contaminated water bio-accumulated as much as 600 ppm Cu until they turn greenish and were still alive [24]. This report may give support to why all the exposed fish survived even at 600 mg/L of Cu treatment. Moreover, all fish exposed to Cu gained weight compared to the control (Figure 4). An indication that Cu ion is required for fish physiological needs [49,50]. However, the tested concentrations for Cu (threshold may differ for cells, animals and plants) in this study may become highly toxic eliciting varying systemic toxicity [50-52]. Intriguing was the fact that fish exposed to mixture of As:Cu significantly gained weight compared to the control, unlike fish exposed to As alone (Figure 4) [53]. It may be suggested that Cu interactively enhance the biotransformation of As into relatively harmless substance hence reducing its deleterious effects during the 7 day exposure [54,55]. Cr, either when exposed to fish alone or in combination with As and Cu caused reduction in fish body weight gain. Decrease in body weight gain was significant for As:Cr and Cr:Cu compared to the control and Cr alone (Figure 4). This suggests that the predicted antagonistic interaction between the binary mixtures of the metals apparently caused a reduction in mortality but did not significantly reduce morbidity (changes in physiological and biochemical processes that retarded fish growth were affected) compared to the negative control. This assertion is supported by the clinical signs of toxicity; ulceration, inflammation, oedema of the skin and skin lesion observed in the fish exposed to As, Cr and As:Cr (Figure 3). Furthermore, the observed skin lesions lend credence to the reports that As and Cr are carcinogens capable of increasing dermatological abnormalities in fish species that may lead to cutaneous and visceral tumor development [56-59]. It further agrees with previous report that long-term human exposure to CCA-treated wood increased cutaneous and visceral malignancy [60]. The derived SC; 6.17 mg/L (Cr), 21.30 mg/L (As), 12.28 mg/L (As:Cr), 10.04 mg/L (Cr:Cu) and 25.46 mg/L (As:Cr:Cu) of the metals derived for juvenile C. gariepinus in this study are higher than permissible concentrations for aquatic environment from Nigeria [61] and other International organizations [62,63], suggesting that the test organism may be safe in the natural environment containing these SCs. However, it is pertinent to consider that anthropogenic activities are constantly and increasingly discharging these metals into both the aquatic and terrestrial environments and they readily accumulate in sediments and aquatic forms. For instance, over 100 folds of the predicted SCs herein have been reported in soil samples (As=1292 mg/kg; Cr=1444 mg/kg; and Cu=924 mg/kg) collected from soil surface where CCA treated woods were placed to dry after treatment [64].
Acute toxicity testing provides evidence of overall toxicity of chemicals via mortality, but does not indicate the possible mechanisms leading to the mortality vis-a-vis alterations in pathophysiology, biochemistry and genetic composition of the test models. Moreover, exposure to sub-lethal concentrations of metals may not elicit instant mortality, therefore it is necessary to evaluate the cumulative risk on organism’s health using appropriate biomarkers [65]. Cytogenetic biomarkers are useful indicators for monitoring impacts of metals and evaluating the risk of predisposition to genetic related diseases and cancer development [66-69]. Fish MN assay, a sensitive and suitable cytogenetic endpoint, has been in use for over 30 decades for monitoring the impact of chemicals on DNA damage [70], making MN and NA formation a suitable and acceptable biomarkers of DNA damage induced by clastogens and aneugens [71]. MN and NA formation in erythrocytes of C. gariepinus has been confirmed sensitive indices for monitoring structural and numerical chromosomal aberrations induced by individual and mixture of chemical and physical agents both in in situ and in vivo studies [32,33,66,72,73].
The differential fold increase in MN and NA erythrocytes in peripheral blood of C. gariepinus exposed to individual and mixture of As, Cr and Cu suggests the varying levels of clastogenic and or aneugenic activities of the metals. The induced MN in exposed C. gariepinus lend credence to the report that Oysters inhabiting a canal with sub-merged CCA-treated woods had two fold higher MN in the gill cells compared to oysters from reference site [25]. Similarly, higher frequency of MN cells were observed in Tradescantia plants cultivated on soil (soil sample analysis; As=1,292 mg/kg, Cr=1,444 mg/kg and Cu=924 mg/kg) where CCA-treated woods were laid to dry after treatment [64]. Moreover, the findings herein that individual metals and their mixtures at their respective 96-h LC50 concentrations induced higher fold increase of MN in fish compared to the positive control (benzene), a known hematogenotoxin [31], is worrisome and suggest management strategies to protect aquatic ecosystems from metal mixture pollution. As and Cr possess the ability to elicit genotoxicity in fish [74,75]. Furthermore, environmental and occupational exposure to these metals had been linked to increased genotoxicity and cancer risk [76-78]. Although, Cu is an essential micronutrient that enhances physiological processes in animals, however it is known to elicit DNA damage when in high concentrations [51,79,80].
Joint-action toxicity among composite mixtures of As, Cu and Cr was evident in the induction of MN and NAs in C. gariepinus. Synergism was observed in lower concentrations (6.25%) of As:Cu and As:Cr while antagonism was the most common interaction among the CCA constituents (Table 5). Previous studies have shown that DNA and protein damage may involve both forms of interactions. For instance, both interactions were observed between lead tetra-acetate and As2O3 in the induction of MN in Tradescantia plant [81]. Synergism between Cu and Zn caused higher MN formation in peripheral erythrocyte of exposed Tilapia species compared to individual Cu and Zn [79]. Also, antagonism between Cu and Zn caused decrease in DNA, RNA and protein damage in fathead minnows, Pimephales promelas than individual Cu and Zn [82]. These interactions depend largely on the toxicokinetics of the metals, which to a greater extent determine changes in the processes involving absorption, distribution, metabolism, and or excretory effects of one metal on the others [83]. The interactive effects of metal mixtures may also depend on the sensitivity of the selected biomarkers of toxicity and the model organisms [84]. The findings herein may suggest that As synergistically interacted with Cu in the exposed fish to enhanced Cu accumulation in the kidney (hematopoietic system of the fish) which led to higher MN and NAs formation via oxidative stress compared to the individual As and Cu. This assertion lends credence to the report of Ademuyiwa et al.[85] wherein they observed that As2O3 increased Cu accumulation in kidney of exposed rats relative to the control group. Furthermore, antagonistic interaction observed herein suggests increase metallothionein production, hence it leads to metal sequestration and decrease free radical formation (ameliorating metal toxicity) [86-88]. This is supported by the report that coexposure between Pb or Cd significantly reduced As accumulation in the kidney of rats, leading to the suppression of oxidative stress [89].
NAs in C. gariepinus along with MN has been shown to be sensitive, reliable and suitable as biomarker of cytogenotoxicity elicited by mixtures of xenobiotics and it suggests possible mechanisms of genome instability in fish [32-34,66,90]. For instance, the presence of nuclear bud has been linked with entrapment of amplified DNA and/or DNA repair complexes during S-phase [91]. While binucleated cell formation results from alterations during cytokinesis of the M phase of dividing cells not previously exposed to cytochalasin-B [92]. Fragmented apoptotic bodies with membrane-bound cells, and cells with loss of membrane integrity (Necrosis) (Figure 5c-d,i-k) suggest that the binary and tri-metal mixtures of CCA constituents caused damage to the hematopoietic system of the exposed fish, and the damage cells were eliminated by either programmed cell death (apoptosis) or accidental cell death (necrosis) [93]. It is also possible that CCA metals induced p53 protein expression which caused activation of antioxidant genes associated with apoptotic cell formation [94]. Cr, Cu and As are capable of generating excess cellular levels of reactive oxygen species which are capable of causing damage to p53 proteins and or genes associated with p53 expression. This can lead to activation of cell death processes; necrosis and apoptosis, via inactivation of antioxidant species [96]. This assertion aligns with the reports that Cr, Cu and As have the ability to interfere with cellular activity through different mechanisms which may lead to cell lysis, cellular inflammation and/or cell death [95]. Necrotic and apoptotic erythrocytes had been previously observed in juvenile C. gariepinus exposed to hospital effluent [33].
Conclusion
In conclusion, joint-action acute toxicity and cytogenotoxic effects of As, Cr and Cu binary and tri-metal composite mixtures showed departure from the actions of individual metals in C. gariepinus. The 96-h acute toxicity showed that Cr was the most toxic metal while Cu and As:Cu were indeterminate. According to Isobologram and SR models, As:Cr and Cr:Cu interacted antagonistically while As:Cu interacted synergistically. Furthermore, concentration-addition model showed synergism for As:Cr:Cu, while the IF deduced that high concentrations of individual metals in the composite mixtures interacted antagonistically to elicit MN and NAs in erythrocytes of exposed fish. The findings herein may suggest possible in situ interactive effects among Cr, Cu and As in contaminated aquatic environment in the elicitation of acute toxicity and cytogenotoxicity in aquatic forms. Increase in genomic instability may predispose fish and other aquatic organisms to genetic related diseases including cancer. The derived SCs of individual and mixture of Cr, Cu and As may be relevant in establishing environmental relevant concentrations / safe limits for the component metals in CCA.
Notes
The authors declare that no form of conflict of interest exists.