Toxicities of Four Parabens and Their Mixtures to Daphnia magna and Aliivibrio fischeri
Article information
Abstract
The objective of this study was to determine toxicities of four parabens (methyl paraben, MP; ethyl paraben, EP; n-propyl paraben, PP; and n-butyl paraben; BP) and their mixtures to two aquatic microorganisms, Daphnia magna and Aliivibrio fischeri. Parabens are one of the widely used preservatives for personal care products, such as cosmetics, pharmaceuticals and food also. First, each paraben was treated to D. magna to measure the toxicity levels as LC20 and LC50. The results showed their value of MP (25.2 mg/L, 73.4 mg/L), EP (18.4 mg/L, 43.7 mg/L), PP (10.4 mg/L, 21.1 mg/L) and BP (3.3 mg/L, 11.2 mg/L). Then, each of the parabens was treated to A. fischeri and calculated their EC20 and EC50 by bioluminescence inhibition test. The results showed the values of MP (2.93 mg/L, 16.8 mg/L), EP (1.18 mg/L, 6.74 mg/L), PP (0.51 mg/L, 5.85 mg/L) and BP (0.21 mg/L, 2.34 mg/L). These four parabens belong to the group classified as being ‘harmful to aquatic organisms’ (above 10 mg/L, below 100 mg/L). After measuring the toxicity, EC20 values of two or more parabens were tested in order to investigate their toxicity. A total of ten combinations of four parabens were tested. As a result, the bioluminescence inhibition test of A. fischeri showed that the toxicity of mixture parabens was stronger than that of a single compound and combinations of three parabens showed the highest bioluminescence inhibition. These results showed that independent toxicity of paraben was maintained. Therefore, it can be predictable that the toxicity of paraben is getting stronger by the addition of other parabens.
INTRODUCTION
Alkyl esters of 4-hydroxybenzoic acid as known as paraben include methyl paraben (MP), ethyl paraben (EP), n-propyl paraben (PP), and n-butyl paraben (BP) are widely used as preservative in personal care products, pharmaceuticals and food [1-3]. Parabens have advantages and disadvantages. Broad spectrum of antimicrobial activity, stability over a wide pH range, solubility in water are advantages of these [4]. On the other hand, a lot of study have suggested that parabens have estrogen mimic activities in vitro and in vivo for mammalian species, and health aspects of these have been focusing for a long time [5-11].
Normally, MP, PP and their mixture are used in a lot of cosmetics and pharmaceuticals. In European Union (EU) countries and Republic of Korea, PP usage in cosmetic products is allowed to 0.4% (w/w) for single ester and 0.8% (w/w) for mixtures of all parabens [12]. In Japan, the maximum concentration of 1% (w/w) parabens are allowed in all cosmetic products [13]. Though some of country in Europe have banned to use it for baby products [14].
Traditionally, aquatic organisms such as crustaceans, fish, and algae are used for aquatic toxicity monitoring [15]. The most popular bioassay used internationally for toxicity screening of chemical compounds and the monitoring of industrial pollutants is certainly the acute toxicity test with freshwater Daphnids such as Daphnia magna and Daphnia pulex [16-22]. In experiments using these organisms, a long exposure time and a large sample volume are required. Such experiments also require difficult skills. Compared to acute toxicity test using freshwater daphnids, bioluminescence inhibition test using A. fischeri requires shorter exposure time, smaller sample volume, easier skills. In addition, it is more cost-effective. For example, according to the manual, the acute toxicity experiment using daphnia requires 24-48 hours of exposure time to an test volume of 50 mL, but the acute toxicity test using bioluminescent bacteria is carried out with 15 to 30 minutes exposure time to a test volume of 200 μL [23]. In this study, these two kinds of acute toxicity tests using D. magna and A. fischeri were conducted to determine toxicities of four parabens and their mixtures to aquatic organism and microorganism [15,24-28].
Parabens are released into the environment from the user and factory wastewater. Because of this, parabens and their salts accumulate in water and become water pollutants. In addition, parabens can penetrate human skin easily. They might accumulate in human cellular tissues. Since parabens are used not only as a single component but also as a mixture, activity of paraben mixtures need to be studied. Many studies have reported toxicities of individual parabens. To the best of our knowledge, no studies have reported toxicities of paraben mixtures up to data. Therefore, the objective of this study is to determine toxicities of four parabens (MP, EP, PP, and BP), which are frequently used in cosmetics, pharmaceuticals, and food, and their mixtures to two aquatic microorganisms (D. magna and A. fischeri).
MATERIALS AND METHODS
Chemicals
The following chemicals were selected for this study: methyl paraben (purity ≥99.0%) ethyl paraben (≥ 99.0%), n-propyl paraben (≥ 99.0%) and n-butyl paraben (≥ 99.0%) were purchased from Sigma–Aldrich (Saint Quentin, Fallaviers, France). These tested chemicals were special-grade reagents and were used without purification. Stock solutions of each paraben were prepared in ethyl alcohol (purity: 99.9%, Samchun, Korea) for the bioluminescence inhibition test of A. fischeri, and in hard reconstituted water (HRW; 0.12 g/L MgSO4, 0.192 g/L NaHCO3, 0.008 g/L KCl, 0.12 g/L CaCO3 in distilled water) for the acute toxicity test of D. magna.
Acute toxicity test of D. magna
In this study, D. magna strain was provided by the Korea Institute of Toxicology (Daejeon, Korea). The organism was cultured according to the U.S. Environmental Protection Agency (EPA) 2002 [23]. The culture work was performed in HRW. Culturing was carried out in an incubator at the temperature of 20±1°C and followed a photoperiod application of 16 hours light: 8 hours darkness. Acute toxicity test was performed according to the standard U.S. EPA manual. Each experiment was performed in triplicate with 10 daphnids in a 50 mL test volume. All the daphnids spent a hour of starvation before treating. To determine the lethal endpoint on D. magna after treating with toxic chemicals, all daphnids were exposed without any feeding until result was checked. After 24 hours, D. magna was counted to calculate the average mortality percentage of each chemical concentration. Then, LC20 and LC50 values at which the mortality is 20% and 50% were determined from regression curves, showing the relationship between the chemicals’ concentrations (mg/L) and mortality of D. magna.
The Inhibition of Bioluminescence of A. fischeri
Luria-Bertani salt medium (LBS; 1% tryptone, 0.5% yeast extract, 2% NaCl, 0.3% glycerol in 50 mM Tris-Cl, pH 7.5) was used for A. fischeri culture. A. fischeri strains were grown at 180 rpm and 30°C until OD600 reached 0.6-0.7. After that, bioluminescence was confirmed over 1 second integration time. All bioluminescence inhibition tests were carried out in white 96-well plate. 2 μL of paraben in ethanol and 198 μL of A. fischeri were transferred into each well, then 200 μL reactants (each concentration of four kinds of parabens in LBS) were treated in each well. 2 μL of ethanol was set as a zero point. Also, each sample was performed in triplicate. The bioluminescence of A. fischeri inhibition was observed in the presence of paraben was measured after different time duration (15 minutes). The bioluminescence was measured by GloMaxⓇ Explorer System (Model GM3500; Promega Corp., Madison, WI, USA). A regression curve showing the relationship between each paraben’s concentrations and bioluminescence inhibition of A. fischeri was used to calculate 20% and 50% effective concentrations (EC20, EC50) for inhibiting bioluminescence of A. fischeri. Relative bioluminescence inhibition rates were calculated using the following equation:
Investigation of Paraben Mixture Toxicity using A. fischeri
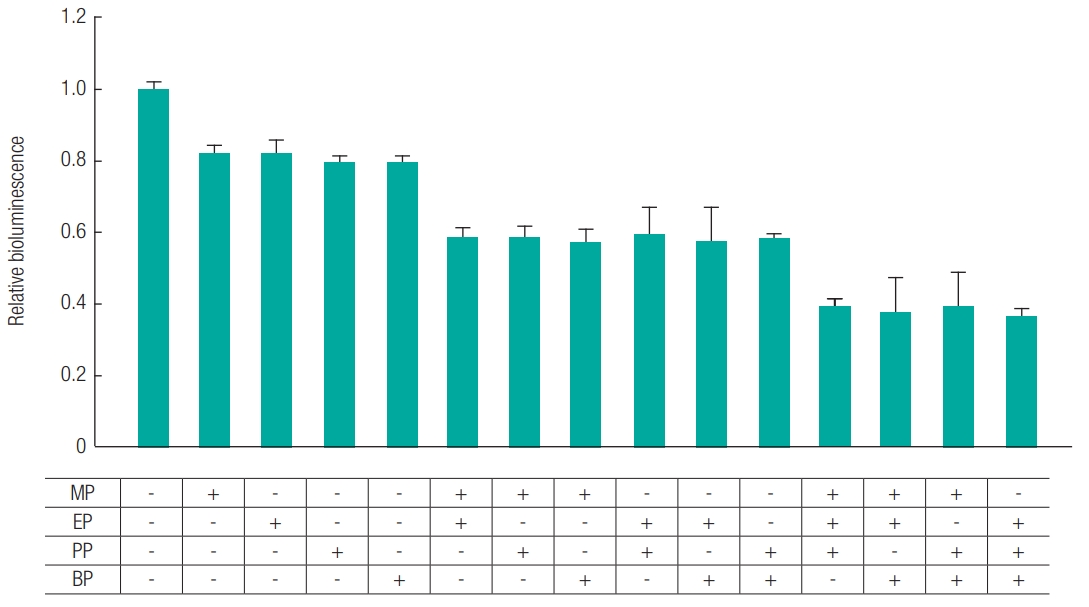
In this part, the bioluminescence inhibition of paraben mixtures was conducted and calculated by the same method as for the bioluminescence inhibition test of A. fischeri. A total of ten combinations of paraben mixtures were examined; MP+EP, MP+PP, MP+BP, EP+PP, EP+BP, PP+BP, MP+EP+PP, MP+EP+BP, EP+PP+BP, and MP+EP+PP+BP. The EC20 value, which did not significantly affect the treatment concentration, was used in mixture [29,30].
RESULTS
LC50 and EC50 are different concepts. The LC50 value entails the kills of 50% population of D. magna. However, EC50 value does not mean killing A. fischeri. To demonstrate this, we confirmed that the growth of A. fischeri was inhibited in the EC50 concentration in the bioluminescence inhibition test However, the difference was not 50%. There was just a slight difference (data not shown).
Acute toxicity tests were conducted using D. magna. The treatment concentration of parabens was from 0 to 300 ppm, which was a range that unaffected and 100% affected, and minimum 10 points of concentration were measured. As shown in Figure 1, the slope has increased sharply as alkyl chain length becomes longer. Therefore, the result shows the length of alkyl chain and the strength of toxicity has positive relationship. The calculated LC50 value for D. magna of parabens ranged from 11.2 mg/L to 73.4 mg/L (Table 1). The toxicity of BP was around seven and two times stronger than that of MP and PP, respectively.
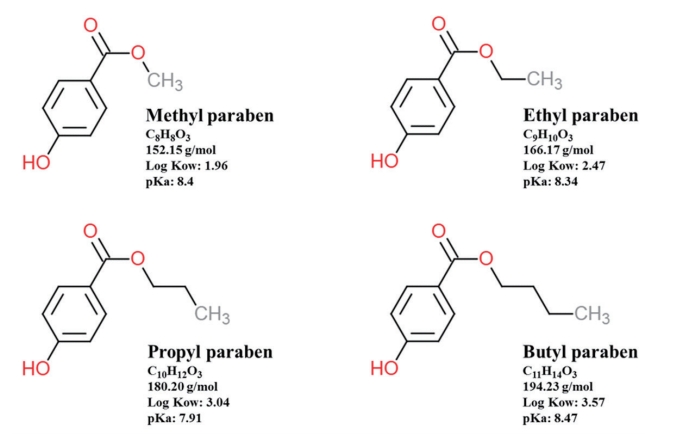
Structure of MP, methyl paraben; EP, ethyl paraben; PP, n-propyl paraben; and BP, n-butyl paraben and their properties.
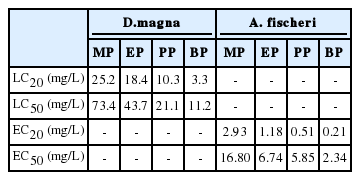
Toxicity parameters obtained using D. magna and A. fischeri, expressed as 20% lethal concentration (LC20; mg/L), 50% lethal concentration (LC50; mg/L), 20% bioluminescence inhibition (EC20; mg/L) and 50% bioluminescence inhibition (EC50; mg/L)
Bioluminescence inhibition test was conducted using A. fischeri. Acute toxicity test of each paraben was performed before the complex effects were examined. The treatment concentration of paraben was from 0 to 50 ppm, which was a range that unaffected and 100% affected, and minimum 8 points of concentration were measured. As shown in Figure 2, relative bioluminescence inhibition rates increase rapidly when alkyl chain gets longer, which has the same tendency as in the acute toxicity test of D. magna. The calculated EC50 value for A. fischeri of parabens ranged from 2.34 mg/L to 16.8 mg/L (Table 1). The toxicity of BP is around seven and two times stronger than that of MP and PP.
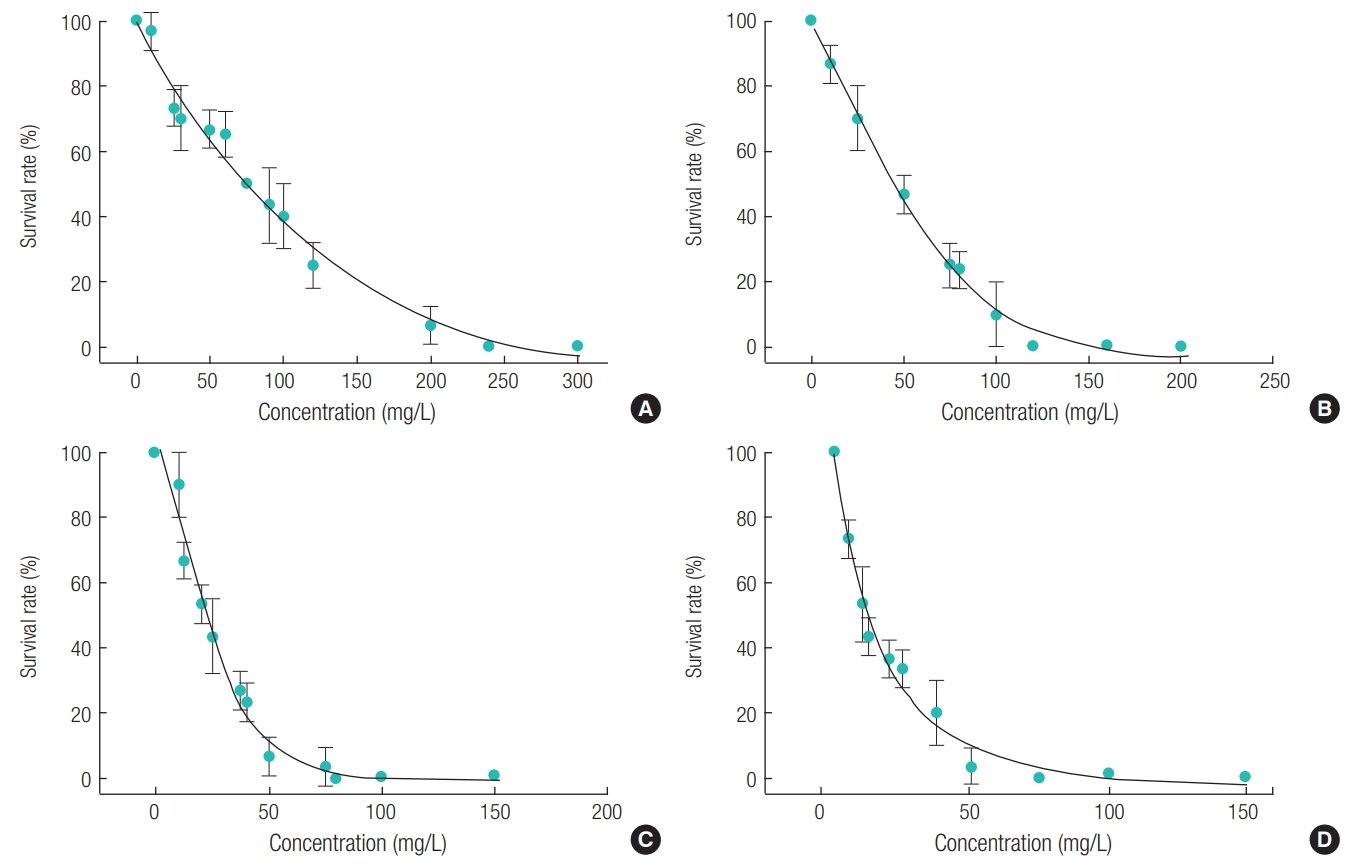
Graph of Acute toxicity to D. magna of: (A) methyl paraben, (B) ethyl paraben, (C) n-propyl paraben, and (D) n-butyl paraben. Survival rate was calculated by counting the number of deaths of D. magna.
To investigate the complex effects of paraben mixtures in EC20 value were calculated from the individual results. The calculated EC20 values were ranged from 0.21 mg/L to 2.93 mg/L (Table 1). Individual toxicity of parabens was maintained in mixture toxicity (Figure 3). For example, acute toxicity of MP (2.93 mg/L: EC20) and PP (0.51 mg/L: EC20) mixture inhibited the intensity of bioluminescence of A. fischeri by 40% (Figure 4). As a result, toxicity was got increased by adding compounds.
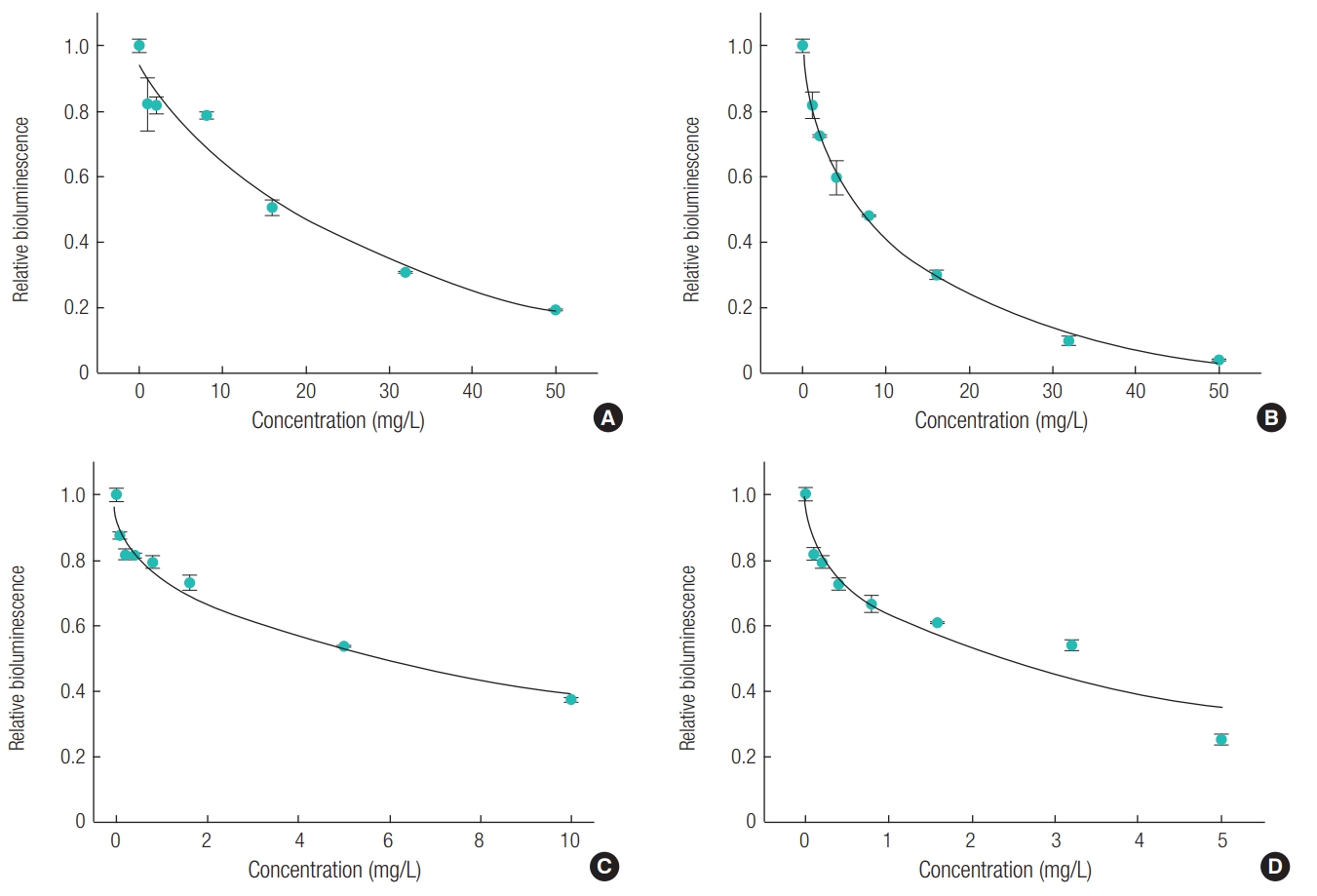
Graph of Acute toxicity to A. fischeri of: (A) methyl paraben, (B) ethyl paraben, (C) n-propyl paraben, and (D) n-butyl paraben.
DISCUSSION
Parabens are used extensively in personal care products, pharmaceuticals and food, and there are several products that use multiple ingredients rather than one. Therefore, it is very likely that various parabens will coexist in the water or in the user’s body. Some studies have been reported that multiple parabens are detected in parts of the body. It is known that the compound is considered to be harmful to aquatic organisms when its LC50 value is greater than 10 mg/L and less than 100 mg/L [31]. Acute toxicity tests with D. magna showed that the LC50 values of four parabens were 11.4 mg/L to 73.4 mg/L, and therefore these parabens could be classified to be harmful to aquatic organisms.
Moreover, the toxicity of each paraben was observed to be stronger as the alkyl chain becomes longer. According to the previous studies, one of the most important factor in determining the toxicity of the parabens is the length of alkyl chain and its solubility [30-35]. As the alkyl chain of paraben becomes longer, the solubility decreases, while the toxicity increases [32-37]. Combined with the results in this study, the length of the alkyl chain and solubility of chemicals result in the increase of the acute toxicity.
Toxicities of parabens to A. fischeri appeared to be higher than those to D. magna. However, this value appeared to be higher than value of A. fischeri, but this is an error in EC and LC interpretation. The LC50 value entails the kills of 50% population of D. magna. However, A. fischeri was hardly affected by growth (data not shown) and only inhibited bioluminescent at concentration of EC50. This can be interpreted that D. magna was affected more sensitive to paraben toxicity than A. fischeri.
As a result of an experiment that the EC20 value of two or more parabens was mixed and treated to A. fischeri, and the inhibition of the bioluminescence was measured to calculate the acute toxicity. all mixtures inhibited bioluminescence by approximately 40%, which indicates mechanism of addition toxicity in mixture parabens. It showed that treatment with twice the EC20 value of a single component inhibited 30%, whereas all mixture has shown higher inhibition that about 10% than single component. As a result, paraben mixture is more toxic in products. Mixtures of MP and PP, which are mainly used in numerous products, showed the same tendency with others.
Mixture of parabens is effective as preservative, while is toxic to aquatic organisms. It is important to determine toxicity of mixture using D. magna, as a higher organism in animals. Moreover, it must determine toxicity of mixture using human cells because all parabens were detected in human body recently. Toxicity of mixtures should be investigated until proper alternative preservative is developed [38,39].
Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No: PJ01267701)” Rural Development Administration, Republic of Korea.