Morphological transformation induced by silver nanoparticles in a Balb/c 3T3 A31-1-1 mouse cell model to evaluate in vitro carcinogenic potential
Article information
Abstract
Carcinogenesis is a complex process involved in genotoxic and non-genotoxic pathways. The carcinogenic potential of silver nanoparticles (AgNPs) has been predicted by examining their genotoxic effects using several in vitro and in vivo models. However, there is no little information regarding the non-genotoxic effects of AgNPs related to carcinogenesis. The in vitro cell transformation assay (CTA) provides specific and sensitive evidence for predicting the tumorigenic potential of a chemical, which cannot be obtained by genotoxicity testing. Therefore, we carried out CTA in Balb/c 3T3 A31-1-1 cells to evaluate the carcinogenic potential of AgNPs. Colony-forming efficiency and crystal violet assays were carried out to determine the cytotoxicity of AgNPs. A cytokinesis-block micronucleus (CBMN) assay and CTA were performed using Balb/c 3T3 A31-1-1 cells to predict the in vitro carcinogenic potential of AgNPs. In the CBMN assay, AgNPs (10.6 μg/mL) induced a significant increase in micronucleus formation indicating a genotoxic effect. Thus, AgNPs could be an initiator of carcinogenesis. In the CTA, used to assess the carcinogenic potential of AgNPs, cells exposed to AgNPs for 72 hours showed significantly induced morphological neoplastic transformation at all tested doses (0.17, 0.66, 2.65, 5.30, and 10.60 μg/mL), and the transformation frequency was significantly increased in a dose-dependent manner. These results indicate that short-term exposure (72 hours) to AgNPs had in vitro carcinogenetic potency in Balb/c 3T3 A31-1-1 cells.
INTRODUCTION
The unique physicochemical properties of nanoparticles have led to their development for biomedical and industrial applications. Among the various nanoparticles, silver nanoparticles (AgNPs) are widely used in industrial and consumer products such as antimicrobial applications, biosensors, composite fibers, cryogenic superconducting materials, cosmetic products, and electronic components [1]. However, the widespread application of AgNPs has led to growing concerns about their potential adverse effects.
In the Integrated Risk Information System (http://www.epa.gov/iris/subst/0099.htm), the carcinogenic potential of silver (Ag) has been reported as Group D (not classified for human carcinogenicity) according to the results of limited animal studies, in which chronic subcutaneous administration of colloidal Ag into rats resulted in an increase in the incidence of malignant tumors [2], but the intramuscular injection of Ag ( < 300 mesh) into rats was not reported to induce cancer [3]. In the nanotechnology field, nanoscale Ag particles with a diameter ranging from 1 to 100 nm have numerous commercial applications. Nanoparticles could have physicochemical characteristics different from those of larger size particle and could induce high reactivity due to their higher surface area [4]. Therefore, it is possible to induce different results with AgNPs in terms of carcinogenicity, as reported in previous studies [2,3].
Carcinogenesis is induced by a multistep pathway, beginning with initiation, promotion, and finally progression [5,6]. Initiation, the first step in carcinogenesis, involves sequential genetic changes induced by DNA damage in a single target cell. Promotion and progression occur via non-genotoxic mechanisms that cause growth alterations and culminate in cells that are able to form malignant tumors [7].
The carcinogenic potential of AgNPs has been predicted by evaluating genotoxic effects using in vitro and in vivo models. AgNPs have been reported to significantly increase micronuclei formation in CHO-K1 cells [8], Syrian hamster embryo (SHE) cells [9], and HepG2 cells [10]. In addition, DNA damage caused by AgNPs has been reported in human microvascular endothelial cells [11] and A549 cells [12], assessed using the comet assay. Jiang et al. [8] reported that DNA adduct and 8-oxo-deoxyguanosine were induced by AgNPs. In animal studies of genotoxicity, albino rats administered repeated intraperitoneal injections containing AgNPs for 28 days showed hepatic histopathological alterations and chromosomal aberrations in bone marrow cells [13]. In addition, rats exposed only to AgNPs showed significantly enhanced micronuclei formation in polychromatic erythrocytes [14]. Li et al. [15] reported that AgNPs reach mouse bone marrow and liver where they have cytotoxic effects on reticulocytes and cause oxidative DNA damage in the liver. Based on these reports, AgNPs could be an initiator of the carcinogenesis processes.
In this study, we used a Balb/c 3T3 A31-1-1 mouse cell model, a major in vitro system for assessing carcinogenesis, to evaluate the carcinogenic potential of AgNPs. Firstly, the Ag nanopowder ( < 100 nm), which was completely dispersed in an experimental medium by sonication, was characterized by energy dispersion X-ray spectrum (EDS) analysis, transmission electron microscopy (TEM), scanning electron microscopy (SEM), dynamic light scattering (DLS), and zeta potential. Secondly, colony-forming efficiency (CFE) and crystal violet (CV) assays were carried out to determine the cytotoxicity of AgNPs. Finally, a cytokinesisblock micronucleus (CBMN) assay and cell transformation assay (CTA) were performed in Balb/c 3T3 A31-1-1 cells to predict the in vitro carcinogenic potential of AgNPs.
METHODS
Materials
Benzo(a)pyrene (B[a]P; CAT No. B1760), anthracene (An; CAT No. A89200), and AgNPs (CAT No. 576832) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Prior to treatment, chemicals (B[a]P and An) were dissolved in dimethyl sulfoxide (DMSO) and stored at -20°C until needed. The final concentration of the vehicle (DMSO) in the assay was adjusted to 0.5% (v/v).
Silver Nanoparticle Sample Preparation and Characterization
Ag nanoparticles (CAT No. 576832; Sigma-Aldrich) were homogeneously dispersed in minimum essential media (MEM) media (Gibco Invitrogen, Grand Island, NY, USA) by sonication for 30 minutes (Bioruptor UCD-200T; Cosmo Bio, Tokyo, Japan), then filtered through a cellulose membrane (0.45-μm pore size; Advantec, Tokyo, Japan). The concentration of filtered AgNPs measured by inductively coupled plasma (Optima 7300 DV; Perkin Elmer, Waltham, MA, USA) was 10.60 ± 0.44 μg/mL. The purity of AgNPs was assessed by EDS analysis (EMAX; Horiba, Kyoto, Japan). TEM ( JEM 2100F; JEOL, Peabody, MA, USA) and SEM (Hitachi, Tokyo, Japan) were used to evaluate the size and shape of AgNPs. The size distribution of AgNPs dispersed in culture medium at the highest concentration (10.60 μg/mL) was measured by a DLS technique Sympatec GmbH Nanophox particle size analyzer (ClausthalZellerfeld, Germany), and the zeta potential was measured using ELS-Z (Otsuka Electronics Inc., Osaka, Japan).
Cell Culture Conditions
Balb/c 3T3 A31-1-1 cells were purchased from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). The cell line (mouse fibroblast cells) were grown in MEM supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), penicillin (100 U/mL), and streptomycin (100 μg/ mL), and were maintained in a humidified incubator at 37°C in a 5% carbon dioxide/95% air atmosphere.
Cytokinesis-block Micronucleus Assay
The CBMN assay was performed to evaluate the genotoxic effects of AgNPs. The Balb/c 3T3 A31-1-1 cells were seeded onto 8-well chamber slides (4 × 104 cells/well) and cultured for 24 hours. The cells were treated with AgNPs at different concentrations (0.17, 0.66, 2.65, 5.30, and 10.60 μg/mL) in culture medium for 24 hours. The cells were washed two times with phosphate buffered saline (PBS) and treated with 1% trisodium citrate for 5 minutes at 4°C. The slides were then placed in fresh fixative (99:1 of methanol: acetic acid) at 4°C and left on a clean bench to air-dry before being placed in ribonuclease A (10 μg/mL in 2 × standard saline citrate (SSC) for 5 minutes at 30°C. The slide was then rinsed with 2 × SSC (0.02 M sodium citrate and 0.3 M sodium chloride) and left on a clean bench to air-dry. After thorough air-drying, the slide was stained with 5% Giemsa solution for 20 minutes. According to the micronucleus (MN) scoring criterion, 500 binucleate cells per independent culture were obtained. To assess the toxicity to cells induced by cytochalasin-B, the cytokinesis-block proliferation index (CBPI) was measured as ([1 × number of mononucleated cells]+[2 × number of binucleated cells]+(3 × number of trinucleated cells])/500 [16].
Range Finding for Crystal Violet Assay and Colonyforming Efficiency Assay
The Balb/c 3T3 A31-1-1 cells (passage 10) at approximately 80% confluency were trypsinized and suspended in M10F complete medium (10% FBS in MEM medium). Then, cells (passage 11) were seeded onto 6-well plates (3 × 103 cells/well/1.5 mL) or a 60 mm dish (200 cells/4 mL) containing M10F complete medium. After 24 hours incubation, the medium was changed to the treatment media (M10F) containing B(a)P (1 μg/mL) as a positive control, An (20 μg/mL) as the negative control, or AgNPs (0.17, 0.66, 2.65, 5.30, or 10.60 μg/mL). Cells exposed to the treatment medium for 3 days were washed two times with PBS, then the medium was replaced with fresh M10F complete medium. After another 3 days (CV) or 5 days (CFE), the cells were fixed and stained with 0.1% CV or 0.04% Giemsa solution to assess cell viability by the CV and CFE assay, respectively. In the CV assay, the stained dye in each well was extracted with 1.5 mL of extraction solution (50:49:1 of ethanol:distilled water:1M hydrogen chloride) and the optical density of each well was measured at 540 nm. In the CFE assay, the stained colonies ( > 50 cells or > 2 mm in diameter) were scored according to image and stereological analysis with an optical system attached to an upright microscope (CX31; Olympus, Tokyo, Japan) and a digital camera (IMTi-solution Inc., Burnaby, BC, Canada).
Cell Transformation Assay
Balb/c 3T3 A31-1-1 cells (passage 10) at approximately 80% confluency were trypsinized and suspended in M10F complete medium. Then, cells (passage 11) were seeded onto a 100-mm dish at a density of 2 × 104 cells/10 mL of M10F complete medium. After 24 hours incubation, the medium was changed to the treatment media (M10F) containing B(a)P, An, or AgNPs. Cells exposed to the treatment medium for 72 hours were washed two times with PBS, then the medium was replaced with 10 mL of fresh Dulbeco’s modified Eagle’s media (DMEM)/FI2 complete medium. Thereafter, DMEM/FI2 medium was replaced every 3 days for a total of 25 days. At day 25, the medium was changed, and cells remained in culture for another 2 days. At day 27, the cells were fixed with methanol for 10 minutes then stained with a 0.04% Giemsa solution for 30 minutes. For the analysis of the CTA, foci consisting of more than about 50 cells or that were more than about 2 mm in diameter were evaluated using a stereomicroscope. Only type III foci were recorded, which are characterized by the following morphological criteria [17]: deep basophilic staining of spindle-shaped cells which were morphologically different to the background monolayer cells, dense multilayering of cells (piling up), random orientation, and invasive growth of cells at the edge of foci (criss-cross pattern). The transformation results were expressed as the transformation frequency (Tf; five replicates per concentration, two experiments performed) using the following formula: Tf = ([A/B × C × D]), where A is the total number of type III foci per treatment; B is CFE (%)/100; C is the plating efficiency (%)/100; D is the number of cells seeded × number of plates; plating efficiency (%) is the number of colonies formed in the control × 100/200, and 200 is the total number of cells seeded into one CFE dish [18].
Statistical Methods
Each assay was performed at least in triplicate. SigmaPlot 12.0 ( Jandel Scentific, San Rafael, CA, USA) and Excel 2010 (Microsoft, Redmond, WA, USA) software were used to analyze the data. The data are expressed as mean ± standard deviation. Statistical analysis was carried out using PASW 18.0 (SPSS Inc., Chicago, IL, USA). Statistical differences between the groups were determined using one-way analysis of variance followed by t-test. Statistical significance was accepted at p< 0.01 or p< 0.05.
RESULTS
Characterization of Silver Nanoparticles
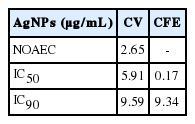
The physicochemical properties of AgNPs dispersed in M10F medium were characterized by EDS, SEM, TEM, DLS, and zeta potential. The EDS analysis to evaluate AgNP purity showed that there were no other impurities present (Figure 1A). In the TEM and SEM analyses (Figure 1B and 1C), AgNPs were observed as spherical aggregates approximately 1-80 nm in size. As shown in Figure 1D and 1E (green box), the cumulative particle size of AgNPs measured by DLS ranged from 1 to 200 nm, and 84% of AgNPs had a diameter of < 100 nm, with an average diameter size for AgNPs dispersed in M10F medium of 80.0 ± 6.0 nm. About 600 particles were counted by SEM (Figure 1D and 1E, light green box), and 73.5% of AgNPs was observed to have a diameter < 100 nm. The zeta potential of the AgNPs was -21.03 ± 2.46 mV in M10F medium (data not shown).
Characterization of silver nanoparticles (AgNPs) by (A) energy dispersion spectrum analysis, (B) transmission electron microscopy, and (C) SEM. The size distribution of AgNPs was measured by DLS and SEM counts (600 particles) of distribution, giving (D) the cumulative volume (%) and (E) size distribution (%). Cts, counts; CBPI, cytokinesis-block proliferation index; SEM, scanning electron microscopy; DLS, dynamic light scattering.
Genotoxic Effects Assessed by Cytokinesis-block Micronucleus Assay
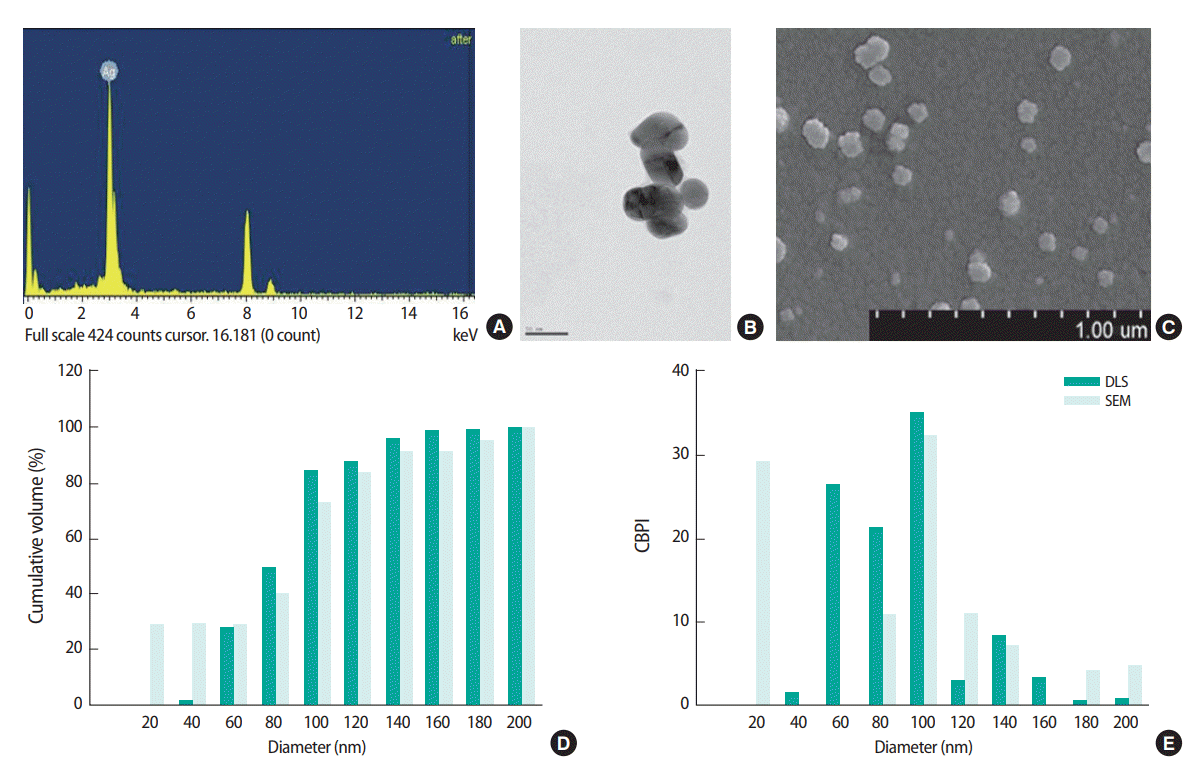
For the CBMN assay, Balb/c 3T3 A31-1-1 cells were exposed to AgNPs at 0.17, 0.66, 2.65, 5.30, or 10.60 μg/mL. These exposure concentrations did not show any cytotoxic effects in the sulforhodamine B and neutral red uptake assays (data not shown). MN formation was significantly increased in cells treated with the highest dose (10.60 μg/mL) of AgNPs, which was increased by about 1.6 times compared to the control. The CBPI was approximately 1.6 in all AgNP-treated groups, but there was no distinct difference between groups except for the positive control (methyl methane sulfonate, 50 μg/mL) (Figure 2).
Micronucleus (MN) formation induced by silver nanoparticles (AgNPs) in Balb/c 3T3 A31-1-1 cells in the presence of cytochalasin B. (A) Micronucleus formation, (B) cytokinesis-block proliferation index (CBPI) index. Cells were treated with AgNPs (0.17, 0.66, 2.65, 5.30, or 10.60 μg/mL) or methyl methane sulfonate (MMS, 50 μg/mL) as the positive control. Results are expressed as the mean±standard deviation of three separate experiments for each data point for MN frequency per 500 binucleate cells. Values are significantly different from the control at *p<0.05 and **p<0.01.
Cytotoxicity Measured by Crystal Violet and Colonyforming Efficiency Assays
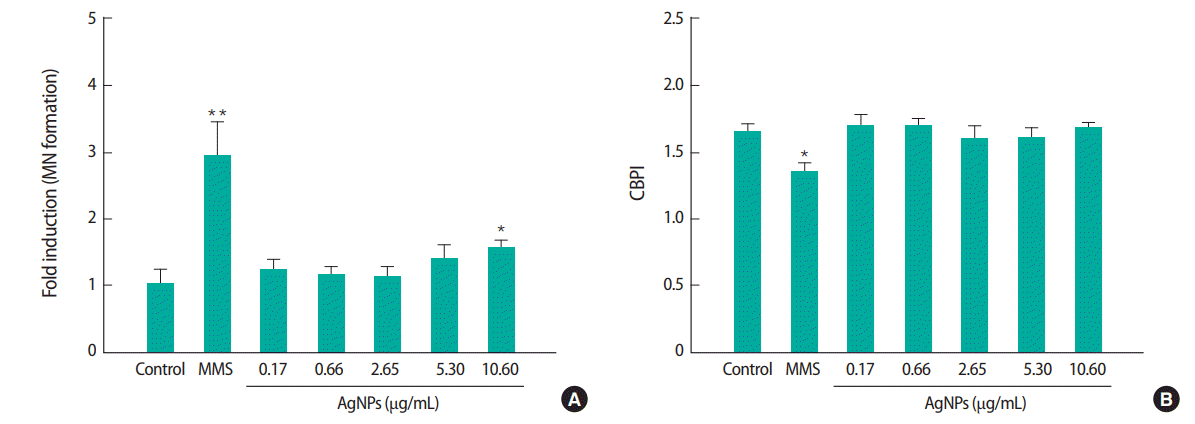
The cytotoxicity of the test compounds was investigated by CV and CFE assays. As assessed by the CV and CFE assay (Figure 3), cell viability was significantly decreased in Balb/c 3T3 A31-1-1 cells exposed to AgNPs in a dose-dependent manner. As shown in Table 1, the concentration resulting in 50% inhibition of cell viability (IC50) and concentration resulting in 90% inhibition of cell viability (IC90) of AgNPs were 5.91 and 9.59, respectively, in the CV assay, while the IC50 and IC90 of AgNPs were found to be 0.17 and 9.34, respectively, in the CFE assay.
Cytotoxic effects of test compounds in the Balb/c 3T3 A31-1-1 cell line assessed by (A) crystal violet (CV) assay and (B) colony-formation assay (CFE). The cells were treated with silver nanoparticles (AgNPs), (0.17, 0.66, 2.65, 5.30, or 10.60 μg/mL), benzo(a)pyrene (B[a]P, 1 μg/mL), or anthracene (An, 20 μg/mL) for 72 hours. The cytotoxic effect of the compounds was presented relative to the control. Results are expressed as the mean±standard deviation of three separate experiments. **p<0.01.
Morphological Transformation
As shown in Figure 4A, the morphological neoplastic transformation of AgNPs was investigated by CTA in Balb/c 3T3 A31- 1-1 cells. The number of foci per plate was significantly increased in all groups exposed to AgNPs compared to the control group. In particular, cells exposed to 0.17 μg/mL AgNPs showed an approximate 3-fold increase in the formation of type III foci compared to the control group. The positive control (B[a]P) showed 21.33-fold increase of foci formation per plate, but the negative control (An) showed no increase in foci formation compared to the control. Figure 4B shows the transformation results (Tf) assessed by the CTA assay, calculated from the type III foci count and cytotoxicity index (CFE). The Tf of 1 μg/mL B(a)P and 10.60 μg/mL AgNPs were 3124.38 ± 198.59 and 1088.53 ± 198.59, respectively.

Morphological neoplastic transformation induced by silver nanoparticles (AgNPs) in Balb/c 3T3 A31-1-1 cells. (A) Number of foci per plate, (B) transformation results (Tf). Cells were seeded onto a 100-mm dish and treated with AgNPs (0.17, 0.66, 2.65, 5.30, or 10.60 μg/mL), benzo(a) pyrene (B[a]P, 1 μg/mL), or anthracene (An, 20 μg/mL). Transformation frequency (Tf) was calculated as indicated in the materials and methods section. Results are expressed as the mean±standard deviation. **p<0.01.
DISCUSSION
According to the European Commission [19], a nanomaterial (NM) must contain at least 50% small particles with a diameter between 1 and 100 nm, which may exist in an unbound state or in an aggregate or agglomerate state. The AgNPs used in our study contained 80% particles smaller than 100 nm in diameter (Figure 1), which means that the AgNPs is being maintained as a NM. The broad use of AgNPs as industrial, household, and healthcare-related products could lead to increased exposure in humans, therefore, further studies on their toxicological effects are required. In this study, we investigated the carcinogenic potential of AgNPs in Balb/3T3 A31-1-1 mouse fibroblasts by CBMN and morphological transformation assays.
Carcinogenesis is a complex process involving both genotoxic and non-genotoxic pathways. Initiation, the first step in carcinogenesis, is related to sequential genotoxic changes induced by DNA damage in a single target cell. Since 2012, genotoxicity studies on AgNPs have shown positive genotoxic effects in several in vitro [8-12] and in vivo [13-15] tests. In the current study, MN formation induced by AgNP exposure (10.60 μg/mL) for 24 hours was increased in the Balb/c 3T3 A31-1-1 mouse fibroblast cell line, as measured by the CBMN assay (Figure 2). The CBMN method has been widely used to evaluate genotoxic effects such as chromosome breakage, impaired DNA repair, chromosome loss, non-disjunction, necrosis, apoptosis, and cytostasis [20]. Similar to our results, exposure to AgNPs (0.01- 10.00 μg/mL) for 24 hours has previously been reported to induce a significant increase in MN formation in BEAS-2B cells [21] and CHO-K1 cells [22], as detected by the CBMN assay. Li et al. [9] found significant MN formation using the CBMN assay in SHE cells exposed to 20 μg/mL AgNPs. In a flowcytometric-based MN assay, AgNPs exposure for 24 hours induced significant MN formation in HepG2 cells (5 μg/mL) [10], CHO-K1 cells (5 μg/mL) [8], and TK6 cells (25 μg/mL) [23]. The effective concentration (10.60 μg/mL) of AgNPs in our study is comparable to that published by others (0.01-20.00 μg/mL). In contrast, Broggi et al. [24] showed a negative impact of AgNPs on MN formation in the same cell line used in our study. In dispersion conditions without the use of a stabilizing agent, the diameter of AgNPs in our study was 80.0 ± 6.0 nm, which is smaller than that reported in the study by Broggi et al. [24] (117.8 ± 16.6 nm). In addition, the effective concentration (10.60 μg/mL) of AgNPs in our study was higher than the exposure concentration (0.11-1.08 μg/mL) of AgNPs reported by Broggi et al. [24]. Therefore, we suggest that the positive results obtained in our study may be due to differences in size and exposure concentration compared to the study by Broggi et al. [24]. Several in vivo MN tests of AgNPs have shown controversial results. In male and female Sprague-Dawley rats, inhalation exposure to AgNPs for 90 days did not have any genotoxic effects on bone marrow [21]. When AgNPs were intravenously injected [14], micronucleated reticulocytes were not observed, although MN formation in the liver was enhanced. In addition, Dobrzyńska et al. [14] reported that AgNPs induced MN formation in polychromatic erythrocytes, but not in reticulocytes and leukocytes. These results indicate that susceptibility to AgNP-induced genotoxicity differ between bone marrow cells and organs. The genotoxicity of AgNPs is a controversial issue, and a clear genotoxic effect of AgNPs has been confirmed in both in vitro and in vivo model systems by us and several other authors. Therefore, we suggest that the genotoxic potential of AgNPs indicates an important role as an initiator of carcinogenesis.
The in vitro CTA can provide specific and sensitive evidence for predicting the tumorigenic potential of a chemical which cannot be supplied by genotoxicity testing [25]. CTA has been considered an alternative in vitro method as it closely models the carcinogenic process of a rodent 2-year carcinogenesis bioassay [26-28]. Therefore, in this study, CTA in the Balb/3T3 A31-1-1 mouse fibroblast cell line was used to evaluate the carcinogenic potential of AgNPs. Firstly, the appropriate dose-range for the transformation assay was based on the data (no observable adverse effective concentration, IC50 and IC90) obtained by the CV and CFE assays [17]. In the current study, the IC50 (0.17 μg/mL) determined by the CFE assay was more sensitive than that determined by the CV assay (5.91 μg/mL), therefore, doses for the morphological transformation experiment were selected based on the results of the CV experiments (Figure 3). At the exposure dose (0.17-10.60 μg/mL), determined based on the CTA results, AgNPs induced a morphological neoplastic transformation (Figure 4). Choo et al. [29] previously reported that long-term exposure to a low-dose (0.13 and 1.33 μg/mL) of AgNPs enhanced in vitro malignant cell transformation in non-tumorigenic BEAS-2B cells. The results of Choo et al. [29] support our results. However, Broggi et al. [24] reported a negative result in regard to morphological neoplastic transformation induced by AgNP exposure (0.11-1.08 μg/mL). Like CBMN result, Broggi et al. [24] used different exposure doses and AgNP sizes than those used in our study. Until now, the carcinogenic potential of AgNPs was a controversial issue. Although the results obtained in the current study confirm the possible role of AgNPs as an initiator and promotor of the carcinogenesis process, these were limited to an in vitro system. Therefore, further animal studies are required to identify any potential carcinogenic effects of AgNPs.
Acknowledgements
This work was supported by a grant from the Korea Ministry of Environment through the Environmental Health R&D Program (2012 001370009) and the National Research Foundation of Korea funded by the Korean government (NRF2013R1A1A2A10060109).
Notes
The authors have no conflicts of interest associated with the material presented in this paper.