Effect of aspect ratio on the uptake and toxicity of hydroxylated-multi walled carbon nanotubes in the nematode, Caenorhabditis elegans
Article information
Abstract
Objectives
In this study, the effect of tube length and outer diameter (OD) size of hydroxylated-multi walled carbon nanotubes (OH-MWCNTs) on their uptake and toxicity was investigated in the nematode Caenorhabditis elegans using a functional mutant analysis.
Methods
The physicochemical properties of three different OH-MWCNTs were characterized. Uptake and toxicity were subsequently investigated on C. elegans exposed to MWCNTs with different ODs and tube lengths.
Results
The results of mutant analysis suggest that ingestion is the main route of MWCNTs uptake. We found that OH-MWCNTs with smaller ODs were more toxic than those with larger ODs, and OH-MWCNTs with shorter tube lengths were more toxic than longer counterparts to C. elegans.
Conclusions
Overall the results suggest the aspect ratio affects the toxicity of MWCNTs in C. elegans. Further thorough study on the relationship between physicochemical properties and toxicity needs to be conducted for more comprehensive understanding of the uptake and toxicity of MWCNTs.
Introduction
Carbon nanotubes (CNTs) are carbon-based nanomaterials that have specific physicochemical and electronic properties [1] that make them applicable to various fields such as lubricating oils, fuel cells, drug delivery systems, and next generation semiconductors [2-4]. CNTs are often functionalized with hydrophilic ligands, as the commercial application of CNTs is often limited by their low solubility and dispersibility in organic or inorganic solutions [5]. To overcome this difficulty, the material surface is functionalized by introducing hydrophilic chemical groups, which lead to higher dispersibility while maintaining the unique properties of pristine CNTs [6]. Functionalized CNTs are therefore widely used as additives, catalysts, sensors, absorbents, intracellular carriers, electrodes, and imaging agents [7,8]. As the use of CNTs in applications and their commercialization have increased, human and environmental exposure to CNTs have also dramatically increased [9,10]. Increased exposure to CNTs has raised concerns over their potential toxicity for human health in the case of occupational and environmental exposure [11]. The potential toxicity of CNTs has been attributed to their physicochemical characteristics including length, diameter, structural defects, surface area, tendency to agglomerate, dispersibility in solution, the presence and nature of catalyst residues, as well as surface chemistry [12-16]. The structure of CNTs is similar to that of asbestos. The similarities in size and shape between CNTs and asbestos fibers have led to concerns about major toxicity of CNTs. Several studies have reported that the high-aspect-ratio (i.e., length divided by diameter) of CNTs can induce pulmonary toxicity [17,18]. As seen with asbestos, high-aspect-ratio multi walled CNTs (MWCNTs) have greater toxicity and potential to induce mesothelioma than low-aspect-ratio MWCNTs [19].
Most of the previous toxicity studies of CNTs have focused on respiratory exposure pathways in vivo or in vitro assays with pulmonary cells, due to the similarity in the structure of CNTs with that of asbestos [19,20]. As potential mechanisms of toxicity of CNTs, most studies emphasize oxidative stress, inflammation, and fibrosis, etc. [20,21]. However, only limited studies are available on the ecotoxicity of CNT [22,23], much less on mechanisms of its toxicity using non-mammalian model species [24,25]. Another research gap in CNT toxicity lies in the potential hazard of functionalized CNTs. Though functionalized CNTs have wide application potential, most CNT toxicity studies have been conducted on pristine CNTs [26,27].
C. elegans is an emerging model in environmental toxicology, both for mechanistic studies as well as high-throughput screening. Various special features such as its well characterized genome, genetic manipulability, ease of maintenance, short and prolific life cycle, transparent and small body size, etc. have led to an increasing use of C. elegans in toxicology [28]. Most of all, a rich collection of available mutants has made it possible to establish mutant screening assays which are not only sensitive and useful in estimating the effects of toxicants but may also shed light on the underlying mechanisms [28,29]. Recently, C. elegans has also been used increasingly as a model for investigating the toxicity of nanomaterials [30].
It has been well documented that physicochemical properties play an important role in the uptake of nanomaterials and subsequently in their toxicity. As described above, aspect ratio is considered an important factor in determining the toxicity of CNTs. Therefore, in this study we investigated whether the outer diameter (OD) size and tube length of hydroxylated (OH)-MWCNTs affected their uptake and toxicity in C. elegans using loss-of-function mutants. Representative genes for uptake and possible toxic mechanism were selected and loss-of-function mutants of these genes were exposed to OH-MWCNTs and their responses compared with that of wildtype. We interpreted the arbitrary response of each mutant compared to wildtype as an indicator of how these genes are involved in the toxicity of different types of MWCNTs.
Materials and Methods
Growth of C. elegans and Treatment with Multi Walled Carbon Nanotubes
C. elegans were grown in Petri dishes on nematode growth medium and fed with OP50 strain Escherichia coli. Worms were incubated at 20°C, with young adults (3 days old) from an age-synchronized culture then being used in all the experiments. wildtype and mutants were provided by the Caenorhabditis Genetics Center (www.CGC.org) at the University of Minnesota. Descriptions of the selected C. elegans mutants are in Table 1.
Multi-wall Carbon Nanotubes
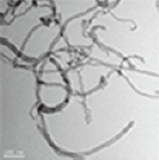
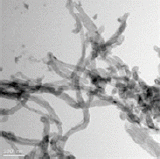
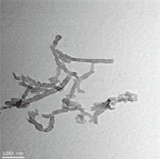
Three different types of OH-MWCNTs were purchased from Cheaptube (www.cheaptube.com). Detailed physicochemical properties of the OH-MWCNTs are presented in Tables 2 and 3. Before exposure to C. elegans, each type of MWCNTs was sonicated for 1 hour using a sonicator (Branson-5210; Branson Inc., Danbury, CT, USA) in distilled water. Starting from stock solutions, experimental concentrations of MWCNTs were prepared in K-medium (0.032 M KCl and 0.051 M NaCl) for C. elegans exposure. The shape and crystal structure of the MWCNTs were determined using a LIBRA 120 transmission electron microscope (TEM; Carl Zeiss, Oberkochen, Baden-Württemberg, Germany). The size distribution and zeta potential of MWCNTs in C. elegans K-media was measured using an electrophoretic light scattering spectrophotometer (ELS; ELS-8000; Otsuka Electronics, Osaka, Japan).

Physicochemical properties of hydroxylated-multi walled carbon nanotubes (OH-MWCNTs) with different sized outer diameters (ODs)
C. elegans Mortality Test
wildtype and mutant C. elegans were exposed to pristine and OH-MWCNTs at a concentration of 500 mg/L in K-media with E. coli OP50 as food. Twenty young adult worms were transferred to 96-well tissue culture plates containing 100 μL of the test solution in each. The worms were exposed for 48 hours at 20°C.
Statistical Analysis
Data are presented in arbitrary units compared to the control, with statistical differences between the wildtype and mutants relating to survival determined by an analysis of variance test, with a Dunnett’s multiple comparison test. All statistical tests were performed with the SPSS version 12.0 KO (SPSS Inc., Chicago, IL, USA).
Results
Outer Diameter Size-dependent Toxicity
Physicochemical Properties
We examined the physicochemical properties of two different type of OH-MWCNTs (Table 2). The OH-MWCNTs had the same tube length (10 to 30 μm) with different OD sizes (Small-OD-MWCNTs, 8 to 15 nm; Large-OD-MWCNTs 20 to 30 nm). The Small-MWCNTs had a greater aspect ratio range (666.7 to 3750) than the Large-MWCNTs (333.3 to 1500). The shape and structure of the MWCNTs were monitored with TEM and their hydrodynamic diameter (HDD) was measured using ELS. Their HDD ranged from 5000 to 9000 nm in K-media. When two different OH-MWCNTs were dispersed in Kmedia, the HDD of the Small-MWCNTs (5000 to 7000 nm) was less than that of the Large-MWCNTs (6000 to 9000 nm). Their dispersity was affected by the structural properties (OD and HDD) of OH-MWCNTs in aqueous phase. The zeta potentials of the MWCNTs, which indicate their ionic stability in aquatic media, were measured in K-media. The OH-MWCNTs had negative zeta potentials, -30.8 mV in the case of Small-MWCNTs and -22.3 mV in the case of Large-MWCNTs.
Mechanism of Uptake and Toxicity
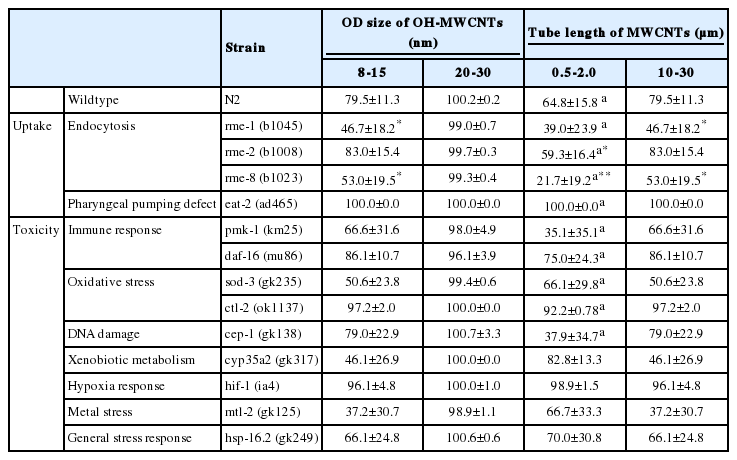
To identify whether the OD sizes of the OH-MWCN affected their uptake and toxicity in C. elegans, we selected loss-of-function mutants of genes involved in uptake (i.e., rme-1, -2, -8 [endocytosis], eat-2 [pharyngeal pumping defect]) and representative toxicity (i.e., sod-3, ctl-2 [oxidative stress], cyp35a2 [xenobiotic metabolism], pmk-1, daf-16 [immune response] cep-1 (DNA damage], hif-1 [hypoxia response], mtl-2 [metal stress], and hsp-16.2 [general stress]) and their responses were compared.
The comparative toxicity of OH-MWCNTs with different ODs showed Small-MWCNTs was more toxic than Large-MWCNTs in wildtype C. elegans (Table 4). Interestingly a smaller HDD was found in K-media exposed to Small-MWCNTs than in media exposed to their Large counterpart (Table 2). Among uptake-related mutants, rme-1 (b1045) and rme-8 (b1023) tended to show a more sensitive response than wildtype in response to Small-MWCNTs exposure, while the toxicity of Big-MWCNTs did not affect. The toxicity by MWCNTs was completely rescued in eat-2 (ad465) mutant, which suggests that ingestion is the main route of MWCNTs uptake.
Survival of wildtype and uptake and toxicity related mutants of C. elegans exposed to multi walled carbon nanotubes (MWCNTs) with different sized ODs and tube lengths
Exposure to Small-MWCNTs led to a decrease in survival for the toxic response related mutant, pmk-1 (km25), sod-3 (gk235), cep-1 (gk138), cyp35a2 (gk317), mtl-2 (gk125), and hsp-16.2 (gk249) comparing to exposure with Big-MWCNTs. Especially, the sod-3 (gk235), cyp35a2 (gk317), and mtl-2 (gk125) mutants were more sensitive than wildtype to Small-MWCNTs exposure.
Tube Length Dependent Toxicity
Physicochemical Properties
Table 3 described the properties of OH-MWCNTs with different tube length. OH-MWCNTs have a same OD size (8 to 15 nm) and different tube length (Short-MWCNTs, 0.5 to 2.0 μm; Long-MWCNTs, 10 to 30 μm). Long-MWCNTs have a higher aspect ratio ranges (666. 7 to 3750) than Short-MWCNTs (33.3 to 250). The shape and structure of MWCNTs were monitored with TEM, the HDD was measured using ELS. When two different OH-MWCNTs were dispersed in K-media, HDD of Short-MWCNTs (700 to 1200 nm) was smaller than that Long- MWCNTs (5000 to 7000 nm). The zeta potentials of MWCNTs had negative zeta potentials, both MWCNTs were observed very similar zeta potentials, -31.5 mV and -30.8 mV.
Mechanism of uptake and toxicity
Comparing the toxicities of Short-MWCNTs and Long-MWCNTs showed that MWCNTs with a shorter tube length were more toxic than those with a longer tube length in wildtype C. elegans (Table 4). In terms of the uptake mechanism, the survival of the eat-2 (ad465) mutant increased when compared with wildtype exposed to Short-MWCNTs and Long-MWCNTs. This result suggests that the possible uptake route of MWCNTs is via ingestion. Among uptake-related mutants, rme-1 (b1045) and rme-8 (b1023) tended to show a more sensitive response than wildtype in response to Short- MWCNTs and Long-MWCNTs.
Among toxic response related mutants, the pmk-1 (km25) and cep-1 (gk138) mutants were more sensitive than wildtype to Short-MWCNTs exposure, whereas sod-3 (gk235), cyp35a2 (gk317) and mtl-2 (gk125) mutants were more sensitive than wildtype to Long-MWCNTs exposure. This result showed that each mutant had a different sensitivity to MWCNTs of different lengths.
Discussion
Many studies have reported that endocytosis/phagocytosis is the cellular uptake mechanism of CNTs [31]. We investigated whether endocytosis was involved in the uptake mechanism of MWCNTs in C. elegans using endocytosis defective mutants such as rme-1,-2,-8. Receptor-mediated endocytosis (RME) is called clathrin-dependent endocytosis as the most common pathway and has been demonstrated to be the internalization mechanism of CNTs [31]. Unexpectedly, all three rme mutants showed a sensitive response to Short-MWCNTs and rme-1 (b1028) and rme-8 (b1023) tended to show a more sensitive response than wildtype in response to Long-MWCNTs (Table 4). Chen et al. [32] reported that amide-single walled CNTs inhibited endocytosis may result in decreased C. elegans growth due to reduced nutrient uptake. Thus, we suggest that endocytosis via the RME pathway may not be involved in the uptake of OH-MWCNTs. In addition, we previously demonstrated phagocytosis as the uptake mechanism of MWCNTs [33]. Finally, toxicity was completely rescued in a pharyngeal pumping mutant, eat-2 (ad465), which strongly suggests that ingestion is the main route of MWCNTs uptake.
We hypothesized that the OD and tube length of MWCNTs plays a role in the toxicity of MWCNTs. Only a few studies have evaluated the role of their diameter in the toxicity of CNTs [14,34]. Nagai et al. [35] compared the toxicity of two different MWCNTs that had similar hydrophobicity, surface reactivity and length, but different diameters (9.4 and 70 nm). They found that the thin MWCNTs were significantly more toxic than the thicker ones, both in vitro and in vivo. Another study also reported that thin MWCNTs showed cytotoxicity and inflammatory and carcinogenic properties. In the current study, the MWCNTs with a smaller OD appeared more toxic than the larger one in wildtype C. elegans (Table 4). Moreover, in the mutant test, the Small-MWCNTs significantly increased the mortality of sod-3 (gk235), cyp35a2 (gk317), and mtl-2 (gk249) mutants compared to wildtype, suggesting that the toxicity of MWCNTs with smaller OD sizes might be related to oxidative stress, xenobiotic metabolism and metal stress. However, further more in depth study would be needed to understand the mechanism.
Regarding the length of MWCNTs, several studies have shown that CNTs with longer lengths and larger diameters have greater toxicity than smaller ones [34]. Another group has also shown that short CNTs do not result in damage to cells. Despite the fact that most studies have shown that longer and wider CNTs lead to greater toxicity, some researchers have found the opposite [36,37]. In the present study, we found the MWCNTs with a shorter tube length were more toxic to wildtype C. elegans; however, mutant analysis provided a hint that the Short- and Long-MWCNTs might exert their toxicities via distinct mechanisms (Table 4). For example, Short-MWCNTs decreased the survival of pmk-1 (km25) and cep-1 (138) mutants compared to wildtype, which represented genes in the mitogen-activated protein kinase pathway and DNA damage repair, respectively, while Long-MWCNTs affected the sod-3 (gk235), cyp35a2 (gk317), and mtl-2 (gk249) mutants. However, here again further study would be needed in order to understand the mechanisms involved.
Aspect ratio (length-to-diameter ratio) is known to affect MWCNT’s toxicity. We therefore investigated toxicity according to the aspect ratio. Many studies have reported that high aspect ratio (long and thin) MWCNTs show more toxicity than low aspect ratio MWCNTs [19,38]. Our results comparing OD sizes support this, as Small-MWCNTs, which had a high aspect ratio, were more toxic than their Large counterpart (Tables 2 and 4). However, the comparison of tube lengths showed the opposite, as the short MWCNTs, which had a low aspect ratio, were more toxic than the long one, which had a high aspect ratio (Tables 3 and 4). In Table 5 we summarize the relationship between aspect ratio and toxicity of CNTs observed here as well as reported previously by other groups. Taken together, CNTs with a high aspect ratio are generally more toxic than those with a low aspect ratio, but this was not always observed, probably due to other factors that affect CNTs toxicity.
Our mutant analysis provides an insight into the mechanism of uptake and toxicity, as we found that each mutant had a different sensitivity different MWCNTs. However, we cannot yet fully explain the variation in the responses of the different mutants. More evidence is needed to fully understand the mechanism of toxicity of MWCNTs.
In conclusion, we have demonstrated that deceasing the OD size and tube length of MWCNTs increased their toxicity to C. elegans, which suggests that diameter and length are important parameters to be considered in CNT-induced toxicity. This and other information will help in designing safer CNTs.
Acknowledgements
This work was supported by a grant from the Korean Ministry of Environment as “Environmental Health R&D Program” (no. 2012001370009). Wildtype (N2) and mutant strains (described in Tables 1) were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Notes
The authors have no conflicts of interest with material presented in this paper.