Construction of a risk assessment system for chemical residues in agricultural products
Article information
Abstract
Objectives
Continuous monitoring of chemical residues in agricultural and food products has been performed by various government bodies in South Korea. These bodies have made attempts to systematically manage this information by creating a monitoring database system as well as a system based on these data with which to assess the health risk of chemical residues in agricultural products.
Methods
Meanwhile, a database system is being constructed consisting of information about monitoring and, following this, a demand for convenience has led to the need for an evaluation tool to be constructed with the data processing system.
Results
Also, in order to create a systematic and effective tool for the risk assessment of chemical residues in foods and agricultural products, various evaluation models are being developed, both domestically and abroad. Overseas, systems such as Dietary Exposure Evaluation Model: Food Commodity Intake Database and Cumulative and Aggregate Risk Evaluation System are being used; these use the US Environmental Protection Agency as a focus, while the EU has developed Pesticide Residue Intake Model for assessments of pesticide exposure through food intake. Following this, the National Academy of Agricultural Science (NAAS) created the Agricultural Products Risk Assessment System (APRAS) which supports the use and storage of monitoring information and risk assessments. APRAS efficiently manages the monitoring data produced by NAAS and creates an extraction feature included in the database system. Also, the database system in APRAS consists of a monitoring database system held by the NAAS and food consumption database system. Food consumption data is based on Korea National Health and Nutrition Examination Survey.
Conclusions
This system is aimed at exposure and risk assessments for chemical residues in agricultural products with regards to different exposure scenarios.
Introduction
Continuous monitoring of chemical residues in agricultural and food products has been performed by government bodies in various countries and by related government bodies in South Korea. These bodies have made attempts to systematically manage this information by creating a monitoring database system and also a system in which to assess the health risk associated with chemical residues in agricultural products based on these data. Furthermore, in 2014, an integrated safety information network was created where related food safety information as well as information related to regulations are systematically shared through a unified management and support system, which has propelled the project to change food safety management from region-based autonomy to a unified country-based center.
Meanwhile, a database system is being constructed consisting of information about monitoring and, following this, a demand for convenience has led to the need for an evaluation tool to be constructed with the data processing system. Also, in order to create a systematic and effective tool for the risk assessment of chemical residues in foods and agricultural products, various evaluation models are being developed, both domestically and abroad. Overseas, systems such as Dietary Exposure Evaluation Model–Food Commodity Intake Database (DEEM-FCID) and Cumulative and Aggregate Risk Eevaluation System (CARES) are being used; these use the US Environmental Protection Agency (EPA) as a focus, while the EU has developed Pesticide Residue Intake Model (PRIMo) for assessments of pesticide exposure through food intake.
In the US, as an effort to assess pesticide residues, DEEM has developed the DEEM-FCID module and the DEEM-Calendex modules, while the US Department of Agriculture’s Continuing Survey of Food Intake by Individuals (USDA’s CSFII) food consumer data is being included to be used as a reference population. CARES, with the support of CropLife America, has been making attempts to evaluate chemical exposure from food, drinking water and various environmental media through intake, inhalation and dermal exposure. CARES is using this information to aid the EPA. These different systems that have been developed have yet to be unified into a comprehensive evaluation method and are being used on a stand-alone basis [1,2].
The current operational conditions of these systems can be compared (Table 1). When setting up the target population with different reference populations, LifeLine uses the birth rate of National Center for Health Statistics (NCHS) as the starting population group. Accordingly, the population group generation is based on US census materials and it can be used by exposure condition. CARES possesses and uses a data set of 100,000 people from the 5% Public Use Microdata Sample/US Census (PUMS) sample material from the US Census. Through this process, CARES secures enough materials for the population sub-group and a representation of the entire US population. In the case of DEEM, it uses investigated individual material in CSFII. Compared to the use of food consumption materials, it can show differences in the secured design of food consumption materials about the reference population. DEEM can be simulated for 7,521,555 (20,607×365 days) days with the consideration of 20,607 people in the reference population group and 365 exposure days. Meanwhile, CARES can be simulated for a total of 36,500,000 days in light of the 100,000 people in the reference group and 365 exposure days [4].
In the case of weight materials, DEEM and CARES use these from CSFII. LifeLine estimates individual height material through a mathematical algorithm of height change with age and the connection between height and weight. Also, LifeLine uses CSFII material which is equivalent to the mathematical algorithm. Each model system uses food recipe material as an important database; this is provided with 9,746 raw agricultural units, depending on the method of cooking in the CSFII survey [4].
Every raw agricultural item is sorted by the US crop group, which is coded by cooked state, food type, and the method of cooking. DEEM and CARES use these data when they conduct risk assessments on raw agricultural products and LifeLine use the database when they conduct risk assessments on food.
Materials and Methods
Domestically, these types of evaluation systems are being developed by the Ministry of Food and Drug Safety, partially through the use of the chemical residue monitoring database or the food consumption database.
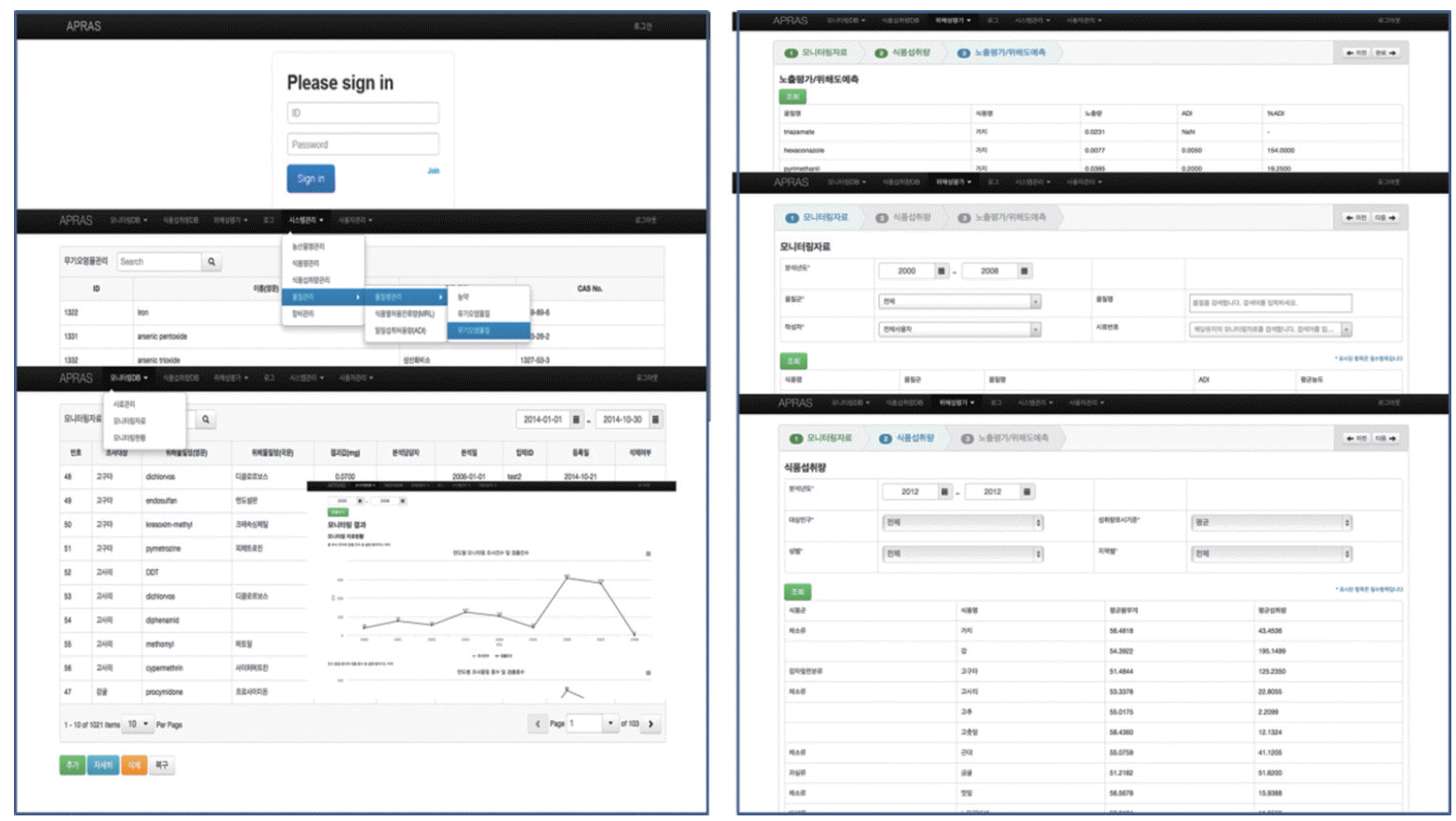
Following this, the National Academy of Agricultural Science (NAAS) created Agricultural Products Risk Assessment System (APRAS), which supports the use and storage of monitoring information and risk assessments. APRAS efficiently manages the monitoring data produced by the NAAS, and creates an extraction feature included in the database system. Also, the database system in APRAS consists of a monitoring database system held by the NAAS and a food consumption database system. Food consumption data are based on Korea National Health and Nutrition Examination Survey (KNHNES). This system is aimed at exposure and risk assessments for chemical residues in agricultural products with regards to different exposure scenarios.
The design of APRAS is more focused on user’s demand to support practice of risk assessment. The system’s main function and structure were designed by the information strategy planning based on results of user demand survey and analysis of work process related setting of regulatory standard. It was considered similar systems overseas (Figure 1).
APRAS recognizes the manager side and the user side. The manager can manage many database-related exposure variables, such as the toxicity baseline value, regulate risk evaluation and adjust the user’s authorization and approval. Users who are given approval by a manager can save and manage their abandoned chemical monitoring material. Furthermore, they can conduct risk evaluations with exposure variable data in the system. Users are able to check the analyzed results of monitoring such as the detection rate or the incongruity rate in the form of a table or chart. The risk evaluation reports are easily checked as they are contained a table and chart and can be used in another report without difficulty. The risk evaluation module is divided into acute exposure evaluations and chronic exposure assessments. In acute exposure assessments, the 97.5% value of food consumption material is utilized [5], the exposure evaluation algorithm is automatically checked depending on the unit weight of the agricultural item and the mode of consumption. It then predicts the exposure rate and makes a choice about the food consumption rate and weight by selected exposed populations in the system.
There are various research materials available on the domestic food consumption rate material. These include food balance sheet data presented by Korean Rural Economic Institute, distribution market data presented by the Korean Food Industry Association, the Korean Food Research Institute, the Korean Health Industry Development Institute, as well as national nutrition survey data from the Korea Centers for Disease Control and Prevention. The national nutrition survey was changed to an annual survey system in 2007. It is composed of a medical examination survey, a health survey, and a nutrition survey. The nutrition survey is composed of a dietary life survey, an infant dietary life survey, a food intake investigation, and a food intake frequency investigation. Food consumption and the food intake frequency data can be used in the exposure evaluation. The food balance sheet and the food distribution market data can be only used for risk assessments at the screening level and as population data, not individual data. This system uses KNHNES data as food consumption data and is constructed on data from 2012. It is planned to extend the data range into the future and the past.
Results
The exposure and risk assessment modules consist of chronic and acute exposure to chemical residues in agricultural products and create various exposure scenarios and exposure factors.
They also contain food consumption rate data, age-specific body weight data provided by the national nutrition survey, and a guide for the application of the acute exposure algorithm for acute exposure assessments based on the unit weight of food.
To conduct the risk evaluation, the toxicity reference value calculates % acceptable daily intake (ADI) based on ADI value and this ADI value is applied to the national standard value. If there is no Korean ADI, risk is predicted with the ADI of Joint FAO/ WHO Expert Committee on Food Additives (WHO/JECFA). The system contains an ADI database which is composed of every country’s ADI information. APRAS is user-friendly system. It shows a summary of used data, exposure scenarios, and exposure evaluation results for each exposure scenario. Also, users can print all of these contents in a report. Because the results from the system are limited when shown on a computer monitor, it can be printed as a report and users can check the summary of the evaluation results on the monitor. Moreover, users can easily check the exposure status of contributions by way of a pie chart and use the results when they make management policies for risk reduction.
Discussion
Exposure to a single substance in different foods can be shown as a sum of the entire exposure value and as aggregate exposure results. It can show common toxicity mechanisms as well. In the case of substance groups which have the same toxicity endpoint, the system contains the substance toxic equivalency factor of these substance groups and conducts a cumulative exposure assessment with the factor.
The system was developed as a web-based application with the accumulation of residue monitoring data and the connectivity of data in mind. This system will aim to include a residue monitoring database on environmental media and to advance for environmental risk assessment.
One of main functions of this system is a probabilistic risk assessment module. This makes the risk value a point estimation but also a distributional estimation. Through this, it can conduct uncertainty analysis as well.
Acknowledgements
This study was supported by Rural Development Administration of the Republic of Korea, under the Joint Research Program (2014).
Notes
The authors have no conflicts of interest with material presented in this paper.