Ingestion Exposure to Nitrosamines in Chlorinated Drinking Water
Article information
Abstract
Objectives
N-Nitrosodimethylamine (NDMA) is classified as a probable human carcinogen by the United States Environmental Protection Agency (US EPA) and is formed during the chlorination of municipal drinking water. In this study, selected nitrosamines were measured in chlorinated drinking water collected from Chuncheon, Kangwon-do, Republic of Korea, and a risk assessment for NDMA was conducted.
Methods
Twelve water samples were collected from 2 treatment plants and 10 household taps. Samples were analyzed for 6 nitrosamines via solid-phase extraction cleanup followed by conversion to dansyl derivatives and high-performance liquid chromatography-fluorescence detection (HPLC-FLD). Considering the dietary patterns of Korean people and the concentration change of NDMA by boiling, a carcinogenic risk assessment from ingestion exposure was conducted following the US EPA guidelines.
Results
NDMA concentrations ranged between 26.1 and 112.0 ng/L. NDMA in water was found to be thermally stable, and thus its concentration at the end of boiling was greater than before thermal treatment owing to the decrease in water volume. The estimated excess lifetime carcinogenic risk exceeded the regulatory baseline risk of 10-5.
Conclusions
This result suggests that more extensive studies need to be conducted on nitrosamine concentration distributions over the country and the source of relatively high nitrosamine concentrations.
INTRODUCTION
Nitrosamines have been found in both chlorinated and chloraminated water. Because many nitrosamines are suspected carcinogens in humans, authorities responsible for supplying drinking water in some countries such as Canada, the United States, and the United Kingdom are concerned about how to assess their adverse health effects on humans and minimize their concentrations [1-6]. N-Nitrosodimethylamine (NDMA) has become a major concern among them because of its frequent occurrence in drinking water and high carcinogenicity [1,2,7].
Both chloramination and chlorination of surface water and groundwater lead to the formation of NDMA through a reaction between monochloramine and an organic nitrogen-containing precursor such as dimethylamine (DMA). However, there is no evidence of DMA sources in natural waters, although DMA in surface water is related to animal wastes and microbial respiration [8]. Probably, ammonia (NH3) in natural waters reacts with chlorine (Cl2), forming chloramines, which can increase the rate of nitrosamine formation [8]. Therefore, it was suggested that ammonia in water need to be removed prior to chlorination to reduce nitrosamine formation [8].
Currently, NDMA is classified as a probable human carcinogen by the United States Environmental Protection Agency (US EPA) and the estimated concentration of 7 ng/L in drinking water corresponds to a lifetime risk of 10-5 [9]. NDMA and five other nitrosamines have been included in proposed Unregulated Contaminant Monitoring Rule 2 (UCMR 2) by the US EPA [10]. Although it is not currently regulated by any federal governments or countries, some states in the United States such as California [11] and a Canadian Province, Ontario [12] have set drinking water notification or action level at 10 ng/L. However, in 2010 the Federal-Provincial-Territorial Committee on Drinking Water of Health Canada proposed a maximum acceptable concentration of 40 ng/L for NDMA in drinking water [7]. In Korea, however, no study has been conducted on the human exposure to nitrosamines or on their concentrations in drinking water. Hence, no regulations on nitrosamines in drinking water were made in Korea.
Chlorinated tap water is frequently boiled to prepare teas, soups, or stews, and the volume of water is reduced during boiling depending on the boiling time. The result is either an increase or decrease in the concentrations of disinfection by-products (DBPs). Either thermally labile or highly evaporative DBPs would be lost from water by boiling, whereas thermally stable and non-volatile compounds would be concentrated due to the volume decrease. Therefore, water-boiling activity can lead to variable human ingestion exposures to water contaminants depending on their properties. For instance, ingestion exposure to trihalomethanes (THMs), including chloroform, from the consumption of hot water is very low or nearly negligible due to their high volatility [13-15]. In contrast, oral exposure to haloacetic acids (HAAs) depends on the types of DBPs. For example, ingestion exposure to dihalogenated HAAs, such as dichloroacetic acid, increases with increasing boiling time due to their thermal stability and the decrease of water volume with time, whereas ingestion exposure to trihalogenated HAAs, such as trihaloacetic acid, decreases with time due to their thermal decarboxylation to their corresponding THMs [13,14].
The dietary patterns of Korean people are unique in that Koreans usually consume large quantity of soups and stews that are boiled for at least 10 minutes during meals. This may cause different exposure to the DBPs contained in tap water compared to drinking unheated water only. However, no information is available on the thermal stability of nitrosamines.
In this study, nitrosamines in chlorinated drinking water samples that were collected from Chuncheon, Kangwon-do, Korea, were measured using solid-phase extraction followed by derivatization and high performance liquid chromatography-fluorescence detection (HPLC-FLD). There after, the two most frequently found nitrosamines were examined for their thermal stability in water. Finally, the risk of the residents from ingestion exposure to NDMA in chlorinated tap water was assessed employing the US EPA's guidelines for carcinogen risk assessment [16].
MATERIALS AND METHODS
I. Materials
Six nitrosamine standards, including N-nitrosomorpholine (NMOR), N-nitrosodimethylamine (NDMA), N-nitrosomethylethylamine (NMEA), N-nitrosopyrrolidine (NPYR), N-nitrosodiethylamine (NDEA), and N-nitrosopiperidine (NPIP) were purchased from Supelco, Inc. (Bellefonte, PA, USA) and the concentration of each standard in an ampoule was 2,000 mg/mL in dichloromethane (DCM; Burdick & Jackson, Morristown, NJ, USA). Each standard was diluted with methanol (Burdick & Jackson) to obtain the desired concentrations and the prepared standard solutions were stored at 4℃. Acetonitrile (Burdick & Jackson, Morristown, NJ, USA) and water (18 MΩ·cm; Shinhan Scientech, Daejeon, Korea) were used as mobile phases. A denitrosating reagent was prepared by adding 1 mL of 48% hydrobromic acid (Wako Pure Chemical Industries, Ltd., Osaka, Japan) in a 10-mL volumetric flask and filling the flask with glacial acetic acid (Daejung Chemical, Siheung, Korea) to the mark. The reagent was stored in a 40-mL amber glass bottle at 4℃ until used within 2 weeks. A dansylating reagent was prepared by dissolving 25 mg of 5-(dimethylamino)naphthalene-1-sulfonyl chloride (dansyl chloride; Calbiochem, Darmstadt, Germany) in acetone (Burdick and Jackson, Morristown, NJ, USA), and diluting to 50 mL (0.5 mg/mL). A pH 10.5 buffer solution was prepared by dissolving 0.32 g of NaOH (Kanto Chemical Co., Inc., Tokyo, Japan) and 2.0 g of NaHCO3 (Daejung Chemical, Siheung, Korea) in water and diluting to 50 mL. n-Hexane and NaCl were obtained from Burdick & Jackson and Daejung Chemical, respectively.
II. Sampling of Chlorinated Drinking Water
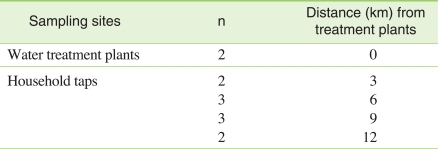
In Chuncheon, a city of Kangwon province, Korea, there are 2 municipal drinking water facilities that have adopted surface water as a drinking water source and chlorination as a means of water disinfection. One water sample was collected from each plant. Two or three samples were obtained from each of 4 sites, which were approximately 3, 6, 9, and 12 km away from the plants, respectively, on May 1, 2009 (Table 1).
Prior to water sampling, pH values (AP 62, Fisher Scientific) and total residual chlorine concentrations (HI 93711, Hanna Instruments) were measured at each site. Water samples were collected in 1-L polypropylene containers to the full after the bottles were thoroughly rinsed 3 times with tap water. Residual chlorine was immediately quenched by adding 0.2 g of sodium thiosulfate into each water sample. Samples were placed in an ice box, transported to the lab, and stored at 4℃ until analyzed within 2 weeks.
III. Thermal Stability of Nitrosamines in Water
Volumes of 500-mL chlorinated tap water were spiked with 15 mL of the NDMA standard solution (2 mg/mL) and NMOR standard solution (5 mg/mL), respectively. The resulting concentrations of NDMA and NMOR in the prepared aqueous solution were 60 and 150 ng/L, respectively. Total residual chlorine (CP-15 Chlorine Photometer; HF Scientific, Fort Myers, FL, USA) and dissolved organic carbon (Sievers 5310C TOC analyzer; GE Analytical Instruments, Boulder, CO, USA) concentrations were measured at the end of each experiment.
Each prepared aqueous solution was placed in a nickel-silver pot and boiled using a gas range for 3 different durations: 5, 10, and 20 minutes. Each experimental set was repeated 3 times. Three control samples were also run at room temperature for 20 minutes. Water volumes were measured to correct for the decrease of volumes after samples were cooled down to room temperature. Sodium thiosulfate (0.2 g) was used to quench residual chlorine. Samples were analyzed for 6 nitrosamines. The following volumes of water were used for the analysis: 460, 450, 360, and 260 mL for control, 5, 10, and 20 minutes, respectively.
IV. Analysis of Water Samples for Nitrosamines
Water samples were analyzed for nitrosamines using the method developed by Cha et al. [17] after some modifications [18]. Nitrosamines in water samples (260 - 500 mL) were extracted using Ambersorb® 572 as a sorbent after pH values were adjusted to 8.2. Eluted nitrosamines were denitrosated and the resulting amines were derivatized to dansyl analogues. Nitrosamines were quantitatively determined using high-performance liquid chromatography-fluorescence detection (HPLC-FLD) with the aid of an autosampler.
The HPLC-FLD system was as follows. Two HPLC pumps (Prostar 210) and the fluorescence detector (model 470) were made by Varian, Inc. (Santa Clara, CA, USA) and Waters Corporation (Milford, MA, USA), respectively. Samples were injected by an autosampler (Prostar model 410, Varian, Inc.). The Microsorb-MV 100-5 C18 column (Varian, Inc.) was used for peak separation, and a mixture of water and acetonitrile (45:55, v/v) was used as a mobile phase flowing at 1 mL/min. Excitation and emission wavelengths for fluorescence were 340 and 530 nm, respectively.
Five-point calibration curves for the quantitative determination of individual nitrosamines were prepared after the matrices spiked with standard solutions were analyzed using the same procedure as that for samples. The coefficients of determination (r2) for the calibration curves were 0.9989, 0.9942, 0.9995, 0.9975, 0.9991, and 0.9951 for NMOR, NDMA, NMEA, NPYR, NDEA, and NPIP, respectively. Method detection limits (MDLs) for respective nitrosamines were estimated to range between 0.51 and 4.4 ng/L based on the signal to noise ratio of 3.
V. Carcinogenic Risk Assessment for NDMA
For NDMA, lifetime average daily doses (LADDs) were estimated using the following equation:
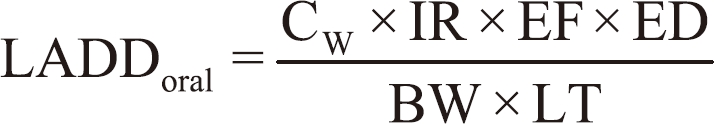
where LADDoral is the lifetime average daily dose from ingestion exposure (mg/kg/day), CW is the concentration of NDMA in chlorinated tap water, IR is the average ingestion rate (1.5 L/day), EF is the exposure frequency (365 days/year), ED is the exposure duration (80.1 years), BW is the body weight of a reference Korean adult (62.8 kg), and LT is the expected lifetime of a Korean person (80.1 years × 365 days/year).
Average, median, and 90th percentile concentrations were used in estimating LADDs. Exposure factors for estimating LADDs were obtained from Korean databases. The average ingestion rate for Korean people was obtained from Jang et al. [19]. Exposure duration and lifetime both were set at 80.1 years [20]. The average adult body weight was set at 62.8 kg [19].
Excess lifetime cancer risks (ELCRs) from ingestion exposure to NDMA were estimated using the oral slope factor (CSF) of 51 (mg/kg/day)-1 available from the US EPA's IRIS site [9] and the following equation:
ELCR = LADD × CSF
ELCRs were estimated for average, median, and 90th percentile NDMA concentrations.
RESULTS AND DISCUSSION
I. Concentrations of Nitrosamines
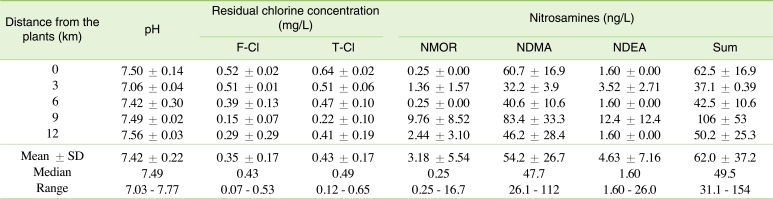
Table 2 summarizes pH values, residual chlorine concentrations, and nitrosamine concentrations measured for chlorinated drinking water samples. The pH values were in the neutral range of 7.03-7.77. Average free and total residual chlorine concentrations at the treatment plants were 0.52 and 0.64 mg/L, respectively, but they were as low as 0.15 and 0.22 mg/L, respectively, at the 9-km sites. The residual chlorine concentrations at the 12-km sites were higher than those at the 9-km sites, because there was a chlorine boosting station at a storage tank between the 2 sites.
Among 6 nitrosamines, 3 compounds including NMOR, NDMA, and NDEA were detected in at least 1 sample. NDMA was the most frequently found, and indeed was found in all samples collected, whereas NMOR and NDEA were found in 41.7 and 25.0% of the total samples, respectively.
NDMA concentrations ranged between 26.1 and 112.0 ng/L. The highest concentration was observed in a sample collected from a 9-km site. However, average NDMA concentrations did not show any decreasing or increasing trend with distance from the treatment plants in this study. In contrast, Charrois et al. [21] and Zhao et al. [22] showed that the concentrations of NDMA and other nitrosamines increased with distance from the treatment plants, which indicates that additional nitrosamines are formed over time. The inconsistency may arise from the complicated water supply network in this area; thus, the distance on the map does not correspond to the traveling distance of treated water.
The NDMA concentrations observed in this study were relatively high compared to previous studies, although only chlorination was used for disinfection. For instance, Charrois et al. [21] found NMOR (2-4 ng/L) and NPYR (1 ng/L) as well as NDMA (2-180 ng/L) in a few finished water samples from 2 cities in Alberta, Canada that use chloramination or chloramination in combination with ultraviolet light (UV) for disinfection. However, Charrois et al. [23] found NDMA only in 1 of 8 chlorinated drinking water samples, with concentrations as low as 12 ± 4 ng/L. Groundwater was their source water. However, NDMA was not observed in the water samples where surface water was used as source water. No information is currently available about source water quality and therefore the reason why the water samples contained such high concentrations is not explainable. More study such as bench-scale chlorination test to confirm the high levels observed from the field sampling is needed.
The measured NDMA concentrations exceeded both the notification and the action level (10 ng/L) set by the state of California in the United States and Ontario, Canada, and the concentration (7 ng/L) corresponding to the 10-5 excess cancer risk estimated by the US EPA's Integrated Risk Information System [9]. Therefore, it is likely that more attention should be paid to nitrosamines in chlorinated municipal water in Chuncheon, and probably in all of Korea, and appropriate actions should be taken to reduce the levels if necessary.
Plumlee et al. [5] recommended reverse osmosis combined with photolysis by UV irradiation as an effective way to remove NDMA and other nitrosamines. Their study showed 59-75% removal efficiency. However, because the decayed products of NDMA photolysis include dimethylamine, nitrite, nitrate, formaldehyde, and formate, additional treatment is necessary to prevent any possible adverse health outcomes from the exposure to the above by-products. Therefore, the removal of NDMA precursors such as ammonia and DMA prior to disinfection procedures such as chloramination or chlorination is preferred to lower the exposure to NDMA and its breakdown by-products.
II. Thermal stability of NMOR and NDMA
NMOR and NDMA showed different thermal stability patterns. The initial volume (500 mL) of water after 5, 10, and 20 min boiling decreased to 495.0 ± 5.0, 401.7 ± 5.8, and 235.7 ± 9.8 mL, respectively. The chlorinated water used for the thermal stability test contained 0.28 mg/L. The average DOC concentrations in water samples decreased with boiling time, corresponding to 12.0, 11.3, 4.25, and 2.10 mg/L for 0, 5, 10, and 20 min boiling, respectively. The total DOC amounts in water samples were calculated by multiplying the DOC concentrations by their respective final water volumes. The total DOC values decreased from an initial value of 5.98 mg to 5.59, 1.71, and 0.49 mg after 5, 10, and 20 min boiling time, indicating that DOC components in the water, including THMs, were lost during boiling [13-15]. Nitrosamines are present at ng/L levels in treated water and thus it is hard to determine whether or not they were lost. However, it is certain from the above result that a large amount of organic components including DBPs are lost by boiling.
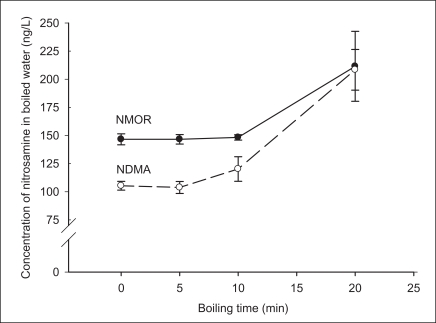
The total amount of NMOR in boiled water did not change significantly in 5-min samples, but apparently decreased with boiling time in 10- and 20-min samples, corresponding to 18.7 and 31.8% decreases, respectively, compared to the initial amount (Figure 1). However, the total amount of NDMA did not vary with time (Figure 1). This suggests that NMOR is thermally labile whereas NDMA is thermally stable. An additional study needs to be conducted on the by-products of the thermal breakdown.

Change in the amount of each nitrosamine in water boiled for different durations.
NDMA: N-itrosodimethylamine , NMOR: N-nitrosomorpholine.
The above results suggest that water boiling during cooking can decrease or increase the concentrations of nitrosamines at the end of boiling, depending on the thermal stability and the quantity of water loss during boiling. As shown in Figure 2, NMOR concentrations apparently did not change up to 10 min of boiling, but greatly increased (44.3% compared to its initial concentration) with 20 min of boiling due to the larger decrease in water volume compared to thermal loss. NDMA concentrations increased more dramatically than NMOR concentrations, rising from 14.1 to 97.8% after 10 and 20 min of boiling, respectively, compared to the initial concentration. These results should be considered in exposure assessments of nitrosamines in tap water in situations where people consume boiled water through beverages, soups, and stews.
III. Risks of chlorinated tap water consumption
LADDs for NDMA were estimated for the average, median, and 90th percentile concentrations, and were 1.42, 1.20, and 2.52 × 10-6 mg/kg/day, respectively (Table 3). Based on these values, ELCRs were estimated to be 7.22, 6.10, and 12.8 × 10-5, respectively (Table 3). These estimates exceeded the baseline risk of 10-5 usually considered for regulatory purposes. To evaluate the NDMA concentration change, it was assumed that whole water (1.5 L/day) is boiled and consumed by Korean people, with 0.8 L of water boiled for 5 min for barley tea, coffee, and other beverages; 0.6 L boiled for 10 min for most soups and stews; and 0.1 L boiled for 20 min for beef-rib soup and beef-rice soup. Using the above assumptions, exposure and risk estimates were approximately 15% lower than those obtained when water boiling was not considered. Therefore, the change in the concentrations of DBPs including nitrosamines needs to be considered when exposure and risk assessments are conducted.
The same is true of other thermally stable DBPs such as dichloroacetic acid; the concentrations of these compounds also increase with boiling time due to the decreasing volume of water with time and the resulting concentration of the compounds [13,14,24]. On the contrary, some DBPs show lowered concentrations after boiling, because they are thermally decomposed into other compounds and/or are lost from water by evaporation because of their high vapor pressures [13,14,24]. An example of such a compound is trichloroacetic acid, which is decomposed to chloroform by thermal treatment. Chloroform is then easily evaporated from water.
CONCLUSIONS
In a Korean city, NDMA in a chlorinated municipal drinking water distribution system was found at relatively high concentrations. Considering the dietary patterns of Korean people, the thermal change of NDMA by boiling was included in the assessments of exposure and risk from the consumption of chlorinated drinking water. NDMA was thermally stable and its total amount was not variable. However, the concentration of NDMA was increased by the reduction in water volume by boiling, leading to elevated ingestion exposure and increased health risk. Using the exposure factors and the potency factor from the US EPA's IRIS, the estimated excess lifetime carcinogenic risk exceeded the regulatory baseline risk of 10-5. The above results suggest that more studies need to be conducted on the nitrosamine concentration distributions in Korea and the source identification of such high levels.
ACKNOWLEDGEMENTS
This work has been financially supported by the Institute of Environmental Research at Kangwon National University.
Notes
The authors have no conflict of interest to declare on this study.
This article is available from: http://e-eht.org/