AbstractRecent studies reported bisphenol A (BPA) and propyl paraben (PrP) are found in human urine, blood, and breast milk samples as well as in food, packaging, socks, and clothes. This means that the two chemicals co-exist in consumer products, and humans are exposed simultaneously to the mixture chemicals. However, the studies on the mixture effects of the two chemicals on human health are not enough. This study was designed to elucidate the effects of orally administered PrP, BPA, and their mixture effects on the uterotrophic response using ovariectomized rats. In addition, the correlation between the uterotrophic response and tissue concentrations of the two chemicals was studied to investigate whether one chemical has any effect on the absorption, distribution, or excretion of the other chemical. Histopathology, hematology, and plasma biochemistry analysis were also performed to evaluate the chemicals’ toxicological effects in the treated rats. Although a significant increase in uterus weight (absolute and relative) was observed in the positive chemical (17β-estradiol) treated group, there were no statistical differences in the uterus weight between the vehicle control and the chemical-treated groups. However, a slight increase in the endometrial glands and a change in the cuboidal to columnar epithelium of the endometrial epithelium were observed in the mixture-treated group. There was no significant toxicity in all treated groups by the hematology and plasma biochemistry analysis results. The results of tissue distribution showed that BPA was mostly detected in the liver while PrP was not detected in most tissues, and the BPA level was higher when the rats were treated with PrP than without PrP, suggesting that PrP may increase the absorption of BPA after oral administration.
IntroductionEndocrine-disrupting chemicals (EDCs) refer to substances that have adverse effects on the endocrine system by interfering with the normal action of hormones. Phthalates, bisphenols, parabens, and UV filters are well-known endocrine disruptors, which are still used or detected as ingredients in various consumer products [1,2,3]. Among them, parabens are used in a wide range of products such as sterilizers and preservatives in cosmetics, pharmaceuticals, personal care products, advertised foods. The endocrine disrupting effects of parabens with various alkyl chains (4-HBA, methyl-, ethyl-, propyl, isopropyl-, butyl-, isobutyl-, benzyl, etc) have been identified through many in vitro studies using cultured HeLa-9903 cells, MCF-7 cells, and MVLN cells [4, 5, 6]. Bisphenol A (BPA) is also widely used as an ingredient in products such as coating agents, plastics, resin, paint, papers, and many consumer products, and many in vitro studies support the endocrine-disrupting effects of BPA [7, 8, 9].
However, in vivo studies of the chemicals, such as uterotrophic assay, are still insufficient and the results are not conclusive. According to a research paper, none of the parabens (methyl-, ethyl-, propyl-paraben) produced any estrogenic response at dose levels up to 100 mg/kg for three days by routes of subcutaneous or oral administration. Notably, ethyl paraben did not induce uterotrophic effects even at a dose level of 1000 mg/kg [10]. But in another study, subcutaneous treatment with parabens for three consecutive days at doses ranging from 5.5 to 210 mg/kg, increased uterine weight in immature Wistar rats although the responses were very weak compared to the 17β-estrogen (E2). The relative uterotrophic potencies related to E2 (100) of these compounds ranged from 0.002 to 0.05 [11]. Although many studies reported evidence of increased estrogen receptor transcription activity in cultured cells, the uterotro
iphic response of parabens using in vivo system is still unclear. In vivo studies on the estrogenic effects of BPA have shown differences in the uterotrophic activity of BPA between oral administration and subcutaneous injection. In most studies, subcutaneous injection has been shown to be more potent than oral administration. When BPA was administered subcutaneously (8, 40, 160 mg/kg/day) or orally (40, 160, 800 mg/kg) for 3 days to immature rats for uterotrophic assay, uterus weights (wet and blotted) were increased in all the subcutaneous groups, but the increase was observed only in 800 mg/kg after oral administration [12].
Recently, Pollock et al published that parabens modulate BPA concentrations in female and male mice [13]. In the study, subcutaneous butyl paraben treatment increased the levels of BPA in blood serum of both sexes, the lung, uterus, and ovaries of females, and the testes and epididymises of males, when BPA was treated as a dietary supplement. Propyl paraben (PrP) also increased the BPA level in the uterus of females. In an Indian report, Varghese et al pointed out that the presence of parabens and bisphenols in maternal products and usage during pregnancy has raised serious concerns about their possible harm to pregnant women [14]. As expected, BPA and PrP were found to co-exist in consumer products such as body lotions, shampoos, conditioners, face lotions, cleansers, and sunscreens. In addition, a mixture of the two chemicals was also detected in various types of vessels [15, 16, 17]. Because PrP and BPA are found in many consumer products, it has been suggested that the two chemicals may have mixture effects on endocrine disruption when exposed simultaneously. However, studies on the mixture toxicity of the two chemicals are not enough, especially in vivo uterotrophic assay.
Based on our previous in vitro study results which showed the mixture effect of PrP and BPA on the ER transcriptional activity [6], an in vivo uterotrophic assay using ovariectomized 7-week-old female Sprague Dawley (SD) rat was performed to evaluate the mixture effects of PrP and BPA after oral administration for three consecutive days. In addition, tissue concentrations of the chemicals were analyzed to investigate if one chemical has effects on the toxicokinetics of the other chemical.
Materials and MethodsTest chemicalsPropyl paraben (PrP) and bisphenol A (BPA) (as test chemicals), corn oil and ethanol (as test chemical solvents), and tert-butyl methyl ether (MTBE), n-hexane, and acetonitrile (as analytical solvents) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 17β-E
estradiol (as a positive chemical) was purchased from the Tokyo chemical industry (Tokyo, Japan). Formaldehyde for tissue fixation was purchased from Fujifilm Wako Pure Chemical Corporation (Osaka, Japan) and methanol (MeOH) for analytical solvent was purchased from Merck Millipore (MA, USA).
Animals and housingOvariectomized 7-week-old female Sprague Dawley (SD) rats were purchased from SLC, Inc. (Hamamatsu, Shizuoka 431–1103, Japan). Animals were randomly assigned to cages with diet and water ad libitum. The animal facility was maintained at 22 °C ± 3 °C temperature, and 50 % ± 10 % relative humidity with a 12 h light/dark cycle. All the animals used in this study were treated in accordance with the Guidance for the Care and Use of Laboratory Animals and approved by the Committee of Dongduk Women’s University (Approval No; 202108-01).
Experimental design and administrationForty SD rats were divided into 8 groups (5 rats per group), and the substances administered to each group are shown in Table 1. In a preliminary test for dose-finding study, two doses of BPA (800 mg/kg and 400 mg/kg) were orally administered to the animals. When rats
mice were treated with 800 mg/kg BPA, serious toxicity were observed and some of the treated animals were found to be dead. However, those toxicity results were not observed in the animals treated with 400 mg/kg BPA (data not shown). Therefore, the highest dose of BPA was set at 400 mg/kg, and a mixture was prepared by mixing BPA with PrP at a ratio of 1:1.
PrP, BPA, and 17β-estradiol are not soluble in water and were first dissolved in ethanol to prepare a high-dose stock. Then the stock was finally diluted with corn oil containing 10% ethanol. The test substances were orally administered once a day for 3 consecutive days at the same time of the day, and the uterus were harvested 24 hours after the last administration of the chemicals. Liver, kidney, and blood sampling were performed at the same time for tissue distribution analysis and toxicological data.
Uterotrophic assayThe uterine weight bioassay procedure used in this study was based on the OECD TG 440: Uterotrophic Bioassay in Rodents, a short-term screening test for estrogenic properties. The animals received daily doses of the test compounds for 3 consecutive days and were sacrificed after 24 h the final administration. The uterus was cut just above its junction with the cervix and the junction of the uterine horn with the ovaries, and was weighed. The body weights of the animals were recorded prior to the administration of the test compounds and immediately before the animals were sacrificed [18].
PrP and BPA analysis in tissues by LC-MS/MSTissue distributions of PrP and BPA were analyzed by LC-MS/MS. For the sample preparation, MTBE:n-hexane (50:50) solvent was added to a certain amount of tissue and homogenized. After that, the sample was centrifuged at 1,000 g for 10 min, at 4 °C, and a portion of the supernatant was taken and centrifuged again at 20,000 g for 10 min, at 4 °C. After a portion of the supernatant was evaporated to dryness, it was re-dissolved in DW:MeOH (50:50) solvent and centrifuged again at the same condition. After a portion of the supernatant was re-evaporated to dryness again, it was re-dissolved in the DW:MeOH (50:50) solvent, and filtered using a 0.2 μm pore-size membrane filter. In the case of plasma, acetonitrile was added and then centrifuged at 1,000 g for 10 min, at 4 °C condition. The acetonitrile fraction was taken and evaporated to dryness, re-dissolved in DW:MeOH (50:50) solvent, and filtered using a 0.2 μm pore-size membrane filter [19, 20].
PrP and BPA were analyzed with high-performance liquid chromatography (Agilent 1200 series HPLC; Agilent Technologies, Santa Clara, CA, USA) coupled with triple quadrupole mass spectrometer (EVOQ Qube™; Bruker Daltonics, Billerica, MA, USA) with C18 column (ZORBAX Eclipse Plus C18; 2.1 mm ⅹ 50 mm, 1.8 μm) (Agilent Technologies, Santa Clara, CA, USA). When analyzing the amount of PrP and BPA, the column temperature was kept at 40 °C and the injection volume was 1 μL. The mobile phase A was 5mM ammonium acetate in deionized water, while mobile phase B was 100% methanol, and mobile phase flow rate was 0.35 mL/min. The gradient started at 10% of solvent B for 3 min, changed linearly to 100% of solvent B in 13 min, and maintained until 18 min. Then, the gradient was set back to the initial percentage of solvent B (10%) after 20 min of LC run, and it was maintained for 3 min for equilibrium. A heated electrospray ionization (HESI) source was used to obtain MS spectra, and the ion source parameters were as follows: spray voltage, 3,500 V; cone temperature, 350 °C; cone gas flow, 20 psi; heated probe temperature, 350 °C; probe gas flow, 45 psi; nebulizer gas flow, 55 psi. Sample introduction and ionization was electrospray ionized in the negative ion mode. Multiple reaction monitoring (MRM) mode was used for the quantitative analysis of PrP and BPA, and the mass transition ion pairs were selected as m/z 179.2→92.2 (17.0 V) and 179.2→136.1(9.0 V) for PrP and m/z 227.1→212.0(12.0 V), 227.1→133.0(19.0 V) and 227.1→93.0(22.0 V) for BPA [21].
HistopathologyThe uterus was collected and fixed in 10% formalin solution. The fixed tissue was trimmed to a thickness of approximately 2 mm ~ 3 mm, put in a cassette, and tissue processing (STP120 Spin tissue Processor, Myr, del Penedès, Spain) was performed for 13 hours. The section was cut into 3 μm ~ 4 μm and attached to the slide. After drying, it was washed with distilled water after deparaffinization and hydration, and stained with hematoxylin and eosin (H&E) [22].
Hematology and plasma biochemistry analysisBlood was collected from each animal 24 hours after the last administration. RBC, HGB, HCT, RBC indices, RDW, MPV, PLT, WBC, and WBC differential counting were analyzed using a hematology analyzer (XN-1000, SYSMEX, Japan). A part of the collected blood was centrifuged (4 °C, 1,000 g for 10 min) to separate plasma. Total protein, albumin, globulin, A/G ratio, AST, ALT, ALP, BUN, creatinine, B/C ratio, total bilirubin, total cholesterol, TG, HDL-C, LDL-C, and CK were analyzed using a chemistry analyzer (AU480, Beckman coulter, US) [23].
Data analysisResults represented the mean ± standard deviation (SD). All statistical analyses were performed using SPSS version 26 (IBM, Armonk, NY, USA) by an independent two treated groups t-test and one-way ANOVA (analysis of variance) for all the treated groups with post-hoc Tukey test. Statistical significance was considered at p < 0.05.
Results and DiscussionUterotrophic effects of PrP, BPA, and their mixtureThe uterus was harvested from the animals, and their blotted weights were recorded (Table 2). The absolute uterus weight of the positive control group (PC) treated with 17β-estradiol was significantly increased to 0.32±0.06 g compared to the vehicle-treated group (VC), 0.12±0.03 g. The relative weight of the uterus to the body was also significantly increased from 0.06±0.01 % in VC to 0.15±0.02 % in PC (p<0.05). However, the absolute and relative weights of the uterus in other chemical-treated groups showed no statistical difference from VC, although the average weight of the uterus in the mixture (PrP and BPA) treated group showed a slight increase, respectively (0.12±0.03 g, 0.07±0.02 %). The increase in uterus weight in the mixture group (high dose) was confirmed by the histological finding in Table 3. The observational findings of the organ structure of the uterus or histological findings which described the cell changes from cuboidal to columnar were evident, although the effect was not significant compared to the 17β-estradiol treated group (Fig.1 and Fig.2).
PrP-only treated groups did not show uterotrophic effects when the animals were treated with oral administration of 200 mg/kg or 400 mg/kg for 3 days in this study. As mentioned before, the uterotrophic responses of PrP are still in discussion [10, 11]. The difference between the exposure routes has also been observed in many other independent studies [24, 25]. The results of a previous study where Sprague-Dawley immature female rats were orally treated with PrP at doses of 62.5, 250, and 1,000 mg/kg, the uterotrophic responses were not observed are similar to our study results. In our study, the PrP doses we selected as 200 mg/kg and 400 mg/kg, and no uterotrophic effect was observed. Generally, the uterotrophic activity of BPA was observed when it was administered subcutaneously [26, 27, 28]. The effective doses of subcutaneous administration once a day for 3 consecutive days ranged from 100 ~ 300 mg/kg. Ashby et al reported that BPA was treated twice a day for 3 consecutive days subcutaneously, uterus weight of the ovariectomized rat was increased even at a low dose of 16.7 mg/rat [26]. A study that compared the effects between oral and subcutaneous exposure routes, showed that the subcutaneous injection of BPA showed positive uterotrophic activity up to a dose of 200 mg/kg, in most experiments. However, no uterotrophic effect was observed in up to 300 mg/kg in the orally treated group [29]. Another study showed that the relative wet/blotted weight of the uterus started to increase from the dose of 800 mg/kg when BPA was orally administered to immature Crj:CD (SD) rats for 3 days [12]. In our study, no effect was shown in both 200 mg/kg and 400 mg/kg treated groups after oral treatment (Table 2). When we mixed PrP and BPA (1:1 by weight) and administered it to the ovariectomized rats (400 mg/kg PrP and 400 mg/kg BPA), the average weight of the uterus was increased compared to the vehicle control group and cell changes from cuboidal to columnar was evident.
Histological examination of uterusHistopathological examination was performed on the vehicle control (VC), the positive control (PC) treated with 17β-estradiol, and the mixture high-dose treated (MH) groups. The results are provided in Fig. 2 and Table 3. As described in Table 3, endometrial epithelial change from cuboidal to columnar, increased number of endometrial glands, and endometrial gland degeneration/necrosis were examined. In the VC group, the endometrial epithelium was cuboidal and the endometrial glands were not developed, which means they were in anestrus. Compared with the VC, the endometrial epithelium of the uterus was changed from cuboidal to columnar epithelium, an increase in endometrial glands, and degeneration and necrosis of the endometrial glands were significantly observed in PC. In the group treated with a mixture high-dose (MH), an increase in the endometrial glands and a change in the cuboidal to columnar epithelium of the endometrial epithelium were mildly observed, suggesting estrogenic effect by the mixture of PrP and BPA.
Luminal epithelium heights, glandular epithelium heights, and myometrium widths increased when PrP was subcutaneously injected at doses of 65 mg/kg and 195 mg/kg for 3 days in ovariectomized mice but no case study was found in orally treated animals [22]. In our study, PrP was administered orally at a dose of 200 mg/kg and 400 mg/kg, respectively. In a study of the uterotrophic assay for BPA using ovariectomized SD rats, estrogen-like histological change was observed in the group treated with 800 (1/6 case) mg/kg by oral administration [30], which is a higher dose compared to our study.
Distributions of test chemicals in the uterus, liver, kidney, and plasmaAs described before, the subcutaneous injection is more efficient in inducing the uterotrophic activity of PrP and BPA compared to oral administration, and the biological response may be related to the tissue concentration in the target organs. In this study, concentrations of PrP and BPA in the liver, kidney, and plasma as well as the uterus were analyzed after oral treatment. PrP was not detected in all the test samples including liver, kidney, plasma, and uterus when rats were sacrificed at 24 h after the last administration of test chemicals (data not shown). PrP was rapidly absorbed within 2 h after single oral ingestion and quickly eliminated in human study. The terminal half-life was 2.9 h [31]. ADME profile of PrP following single oral or subcutaneous doses at 100 mg/kg to SD rats, plasma Cmax and AUC values were 4- to 10- fold higher compared to dermal administration. Following the oral or subcutaneous administration, urinary excretion was predominant and more than 70% was excreted during the first 24 h, and less than 2 % was retained in the tissues and carcasses [32]. When tissues were collected 24 h after oral treatment, analyses showed that PrP retained in the liver, kidney, uterus, and plasma was undetectable in this study. It seems to be due to the quick elimination of PrP after oral administration, as described previously.
The concentrations of BPA were analyzed after oral administration (Fig. 3). The detected concentrations of the BPA in a low dose of BPA treated group (BPL) and in a low-dose mixture-treated group (ML) were 2.9±1.5 μg/g and 3.8±1.4 μg/g, respectively, and those in high dose of BPA treated group (BPH) and in high dose of mixture treated group (MH) were 3.7±1.5 μg/g and 5.8±2.4 μg/g in the liver, respectively. BPA levels in tissue were increased when rats were treated with PrP, compared to without PrP. Similarly, BPA concentrations in some organs were increased when PrP was administered first and then a fixed concentration of 14C-BPA was administered. Tyler et al investigated whether PrP exposure can modulate the concentration of BPA and 17b-estradiol. When mice were given a subcutaneous injection of PrP, then given a dietary supplement containing BPA, PrP significantly elevated BPA concentrations in the uterus. This suggests that PrP can alter toxicokinetics of BPA by inhibiting the enzyme activity that is critical for the metabolism of BPA [13].
Hematology, plasma biochemistry, and organ/body weightBlood was collected from the test animals 24 h after the last administration of the test chemicals, and then hematology and plasma biochemical analysis were performed (Table 4 and Table 5). The results of the hematologic examination showed that there were no statistical differences by PrP, BPA and their mixtures except monocyte level which was elevated in high dose of PrP treated group. However, this did not seem to have originated from the test chemicals and does not have any toxicological meaning. In addition, the plasma biochemical examination results showed that there were no meaningful toxicological effects by the test chemicals even though the statistical differences were shown in ALT, total bilirubin, and triglyceride. There were no statistical differences in the absolute and relative weight of livers and kidneys between control group and treated groups (Table 2).
ConclusionsUterotrophic response was examined after oral administration of PrP, BPA, and their mixture for 3 consecutive days using ovariectomized rats. The average weight of the uterus and the liver distribution level of BPA were increased in the mixture-treated group, but there was no statistical significance. In histopathological results, the mild change from cuboidal to columnar and increased number of endometrial glands were observed in the mixture group. No other toxicological effects of the mixtures were observed in hematology and plasma biochemistry. In conclusion, the in vivo uterotrophic response by oral treatment of bisphenol A was not observed in both 200 mg/kg and 400 mg/kg doses but the effect seemed to be potentiated by propyl paraben, and further study would be necessary.
AcknowledgementThis work was supported by Korea Environment Industry & Technology Institute (KEITI) through Technology Development Project for Safety Management of Household Chemical Products, funded by Korea Ministry of Environment (MOE)(2020002970001)
NotesCRediT author statement
JP: Animal test, Chemical analysis, Writing-Original draft preparation; HL: Animal test, DC: analysis of PrP and BPA, KP: Supervision, Writing, Review & Editing, Project management.
References1. Virant-Klun I, Imamovic-Kumalic S, Pinter B. From oxidative stress to male infertility: review of the association of endocrine-disrupting chemicals (Bisphenols, Phthalates, and Parabens) with Human Semen Quality. Antioxidants (Basel) 2022;11(8):1617 https://doi.org/10.5620/10.3390/antiox11081617.
2. Koniecki D, Wang R, Moody RP, Zhu J. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res 2011;111(3):329-336 https://doi.org/10.1016/j.envres.2011.01.013.
3. Park JH, Kim JP, Kim JA, Seo KW, Kim ES, Seo JM. Survey of preservatives and UV filter ingredients of distributed sunblock products in Korea. Journal of the Society of Cosmetic Scientists of Korea 2017;43(4):381-390 https://doi.org/10.15230/SCSK.2017.43.4.381.
4. Soni MG, Carabin IG, Burdock GA. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol 2005;43(7):985-1015 https://doi.org/10.1016/j.fct.2005.01.020.
5. Wei F, Cheng H, Sang N. Comprehensive assessment of estrogenic activities of parabens by in silico approach and in vitro assays. Sci Total Environ 2022;845: 157194 https://doi.org/10.1016/j.scitotenv.2022.157194.
6. Lee H, Park J, Park K. Effects of consumer products chemicals ingredients and their mixtures on the estrogen receptor/androgen receptor transcriptional activation. Chemosphere 2022;302: 134866 https://doi.org/10.1016/j.chemosphere.2022.134866.
7. Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol 2011;127(1–2):27-34 https://doi.org/10.1016/j.jsbmb.2011.05.002.
8. Skledar DG, Mašič LP. In vitro estrogenic activity of binary and multicomponent mixtures with bisphenol A. Sci Total Environ 2020;707: 135211 https://doi.org/10.1016/j.scitotenv.2019.135211.
9. Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, et al. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci 2005;84(2):249-259 https://doi.org/10.1093/toxsci/kfi074.
10. Hossaini A, Larsen JJ, Larsen JC. Lack of oestrogenic effects of food preservatives (parabens) in uterotrophic assays. Food Chem Toxicol 2000;38(4):319-323 https://doi.org/10.1016/s0278-6915(99)00160-x.
11. Lemini C, Jaimez R, Avila ME, Franco Y, Larrea F, Lemus AE. In vivo and in vitro estrogen bioactivities of alkyl parabens. Toxicol Ind Health 2003;19(2–6):69-79 https://doi.org/10.1191/0748233703th177oa.
12. Yamasaki K, Sawaki M, Takatsuki M. Immature rat uterotrophic assay of bisphenol A. Environ Health Perspect 2000;108(12):1147-1150 https://doi.org/10.1289/ehp.001081147.
13. Pollock T, Weaver RE, Ghasemi R, deCatanzaro D. Butyl paraben and propyl paraben modulate bisphenol A and estradiol concentrations in female and male mice. Toxicol Appl Pharmacol 2017;325: 18-24 https://doi.org/10.1016/j.taap.2017.04.001.
14. Varghese B, Jala A, Das P, Borkar RM, Adela R. Estimation of parabens and bisphenols in maternal products and urinary concentrations in Indian pregnant women: daily intake and health risk assessment. Environ Sci Pollut Res Int 2022;29(15):21642-21655 https://doi.org/10.1007/s11356-021-17298-5.
15. Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspect 2012;120(7):935-43 https://doi.org/10.1289/ehp.1104052.
16. Gao CJ, Kannan K. Phthalates, bisphenols, parabens, and triclocarban in feminine hygiene products from the United States and their implications for human exposure. Environ Int 2020;136: 105465 https://doi.org/10.1016/j.envint.2020.105465.
17. Herrero L, Quintanilla-López JE, Fernández MA, Gómara B. Plasticisers and preservatives in commercial milk products: A comprehensive study on packages used in the Spanish market. Food Chem 2021;338: 128031 https://doi.org/10.1016/j.foodchem.2020.128031.
18. OECD Test (440): Uterotrophic Bioassay in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing; Paris: 2007. https://doi.org/10.1787/9789264067417-en.
19. Van Overmeire I, Vrijens K, Nawrot T, Van Nieuwenhuyse A, Van Loco J, Reyns T. Simultaneous determination of parabens, bisphenols and alkylphenols in human placenta by ultra-high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2019;1121: 96-102 https://doi.org/10.1016/j.jchromb.2019.05.012.
20. Zhao Y, Liu G, Shen H, Shen JX, Aubry AF, Sivaraman L, et al. Bioanalysis of propylparaben and p-hydroxybenzoic acid, and their sulfate conjugates in rat plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2014;947–948: 68-74 https://doi.org/10.1016/j.jchromb.2013.12.012.
21. Lee N, Jang DY, Lee DH, Jeong H, Nam KT, Choi DW, et al. Local toxicity of biocides after direct and aerosol exposure on the human skin epidermis and airway tissue models. Toxics 2021;9(2):29 https://doi.org/10.3390/toxics9020029.
22. Lemini C, Hernández A, Jaimez R, Franco Y, Avila ME, Castell A. Morphometric analysis of mice uteri treated with the preservatives methyl, ethyl, propyl, and butylparaben. Toxicol Ind Health 2004;20(6–10):123-32 https://doi.org/10.1191/0748233704th202oa.
23. Han ZZ, Xu HD, Kim KH, Ahn TH, Bae JS, Lee JY, et al. Reference data of the main physiological parameters in control Sprague-dawley rats from pre-clinical toxicity studies. Laboratory Animal Research 2010;26(2):153-164.
24. Routledge EJ, Parker J, Odum J, Ashby J, Sumpter JP. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol 1998;153(1):12-19 https://doi.org/10.1006/taap.1998.8544.
25. Vo TT, Jeung EB. An evaluation of estrogenic activity of parabens using uterine calbindin-d9k gene in an immature rat model. Toxicol Sci 2009;112(1):68-77 https://doi.org/10.1093/toxsci/kfp176.
26. Ashby J, Odum J, Paton D, Lefevre PA, Beresford N, Sumpter JP. Re-evaluation of the first synthetic estrogen, 1-keto-1,2,3, 4-tetrahydrophenanthrene, and bisphenol A, using both the ovariectomised rat model used in 1933 and additional assays. Toxicol Lett 2000;115(3):231-238 https://doi.org/10.1016/s0378-4274(00)00198-3.
27. Goloubkova T, Ribeiro MF, Rodrigues LP, Cecconello AL, Spritzer PM. Effects of xenoestrogen bisphenol A on uterine and pituitary weight, serum prolactin levels and immunoreactive prolactin cells in ovariectomized Wistar rats. Arch Toxicol 2000;74(2):92-98 https://doi.org/10.1007/s002040050658.
28. Kim HS, Han SY, Yoo SD, Lee BM, Park KL. Potential estrogenic effects of bisphenol-A estimated by in vitro and in vivo combination assays. J Toxicol Sci 2001;26(3):111-118 https://doi.org/10.2131/jts.26.111.
29. Tinwell H, Joiner R, Pate I, Soames A, Foster J, Ashby J. Uterotrophic activity of bisphenol A in the immature mouse. Regul Toxicol Pharmacol 2000;32(1):118-126 https://doi.org/10.1006/rtph.2000.1412.
30. Conley JM, Hannas BR, Furr JR, Wilson VS, Gray LE Jr. A demonstration of the uncertainty in predicting the estrogenic activity of individual chemicals and mixtures from an in vitro estrogen receptor transcriptional activation assay (t47d-kbluc) to the in vivo uterotrophic assay using oral exposure. Toxicol Sci 2016;153(2):382-395 https://doi.org/10.1093/toxsci/kfw134.
31. Shin MY, Shin C, Choi JW, Lee J, Lee S, Kim S. Pharmacokinetic profile of propyl paraben in humans after oral administration. Environ Int 2019;130: 104917 https://doi.org/10.1016/j.envint.2019.104917.
32. Aubert N, Ameller T, Legrand JJ. Systemic exposure to parabens: pharmacokinetics, tissue distribution, excretion balance and plasma metabolites of [14C]-methyl-, propyl- and butylparaben in rats after oral, topical or subcutaneous administration. Food Chem Toxicol 2012;50(3–4):445-454 https://doi.org/10.1016/j.fct.2011.12.045.
Figure 1Representative uterus from rats treated with test substances. Rats were treated with test materials for 3 consecutive days by oral administration. The rat uterus was excised and weighed 24 h after the last treatment. The representative uterus of each group is shown. VC; vehicle control (corn oil with 10% ethanol), PC; positive control (0.3 mg/kg 17β-estradiol), BPL; bisphenol A 200 mg/kg, BPH; bisphenol A 400 mg/kg, PPL; propyl paraben 200 mg/kg, PPH; propyl paraben 400 mg/kg, ML; mixture of propyl paraben (200 mg/kg) and bisphenol A (200 mg/kg), MH; mixture of propyl paraben (400 mg/kg) and bisphenol A (400 mg/kg). 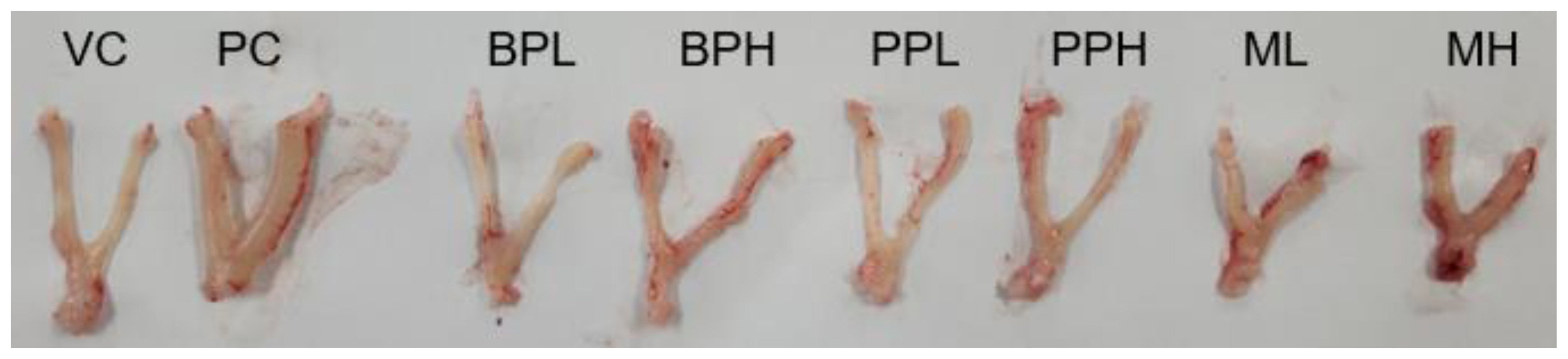
Figure 2Representative histopathologic photographs of the uteruses after hematoxylin and eosin (H&E) staining. Rats were treated with test materials for 3 consecutive days by oral administration. The rat uterus was excised and fixed in formalin solution 24 h after the last treatment. The endometrial epithelium of the uterus was cuboidal in the vehicle control group (VC) while the endometrial epithelium was changed from cuboidal to columnar epithelium in the PC and MH groups (arrow). Degeneration and necrosis of the endometrial glands (arrowhead) were observed in PC. VC; vehicle control (corn oil with 10% ethanol), PC; positive control (0.3 mg/kg 17β-estradiol), MH; mixture of propyl paraben (400 mg/kg) and bisphenol A (400 mg/kg). The number means the magnification ratio. The scale bar is 100 μm. 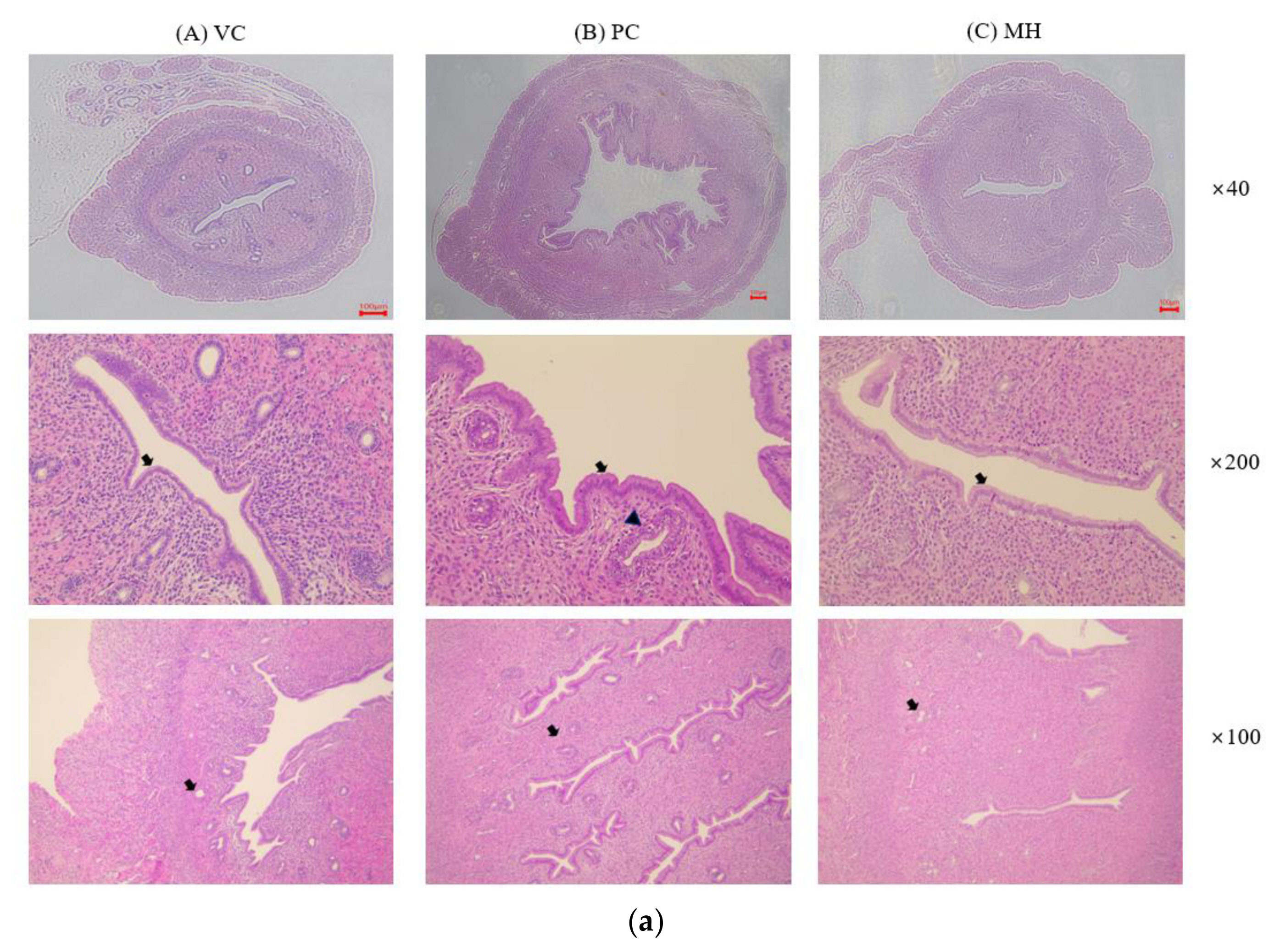
Figure 3Tissue distribution level of bisphenol A in rats. Rats were treated with test materials for 3 consecutive days by oral administration. Plasma and tissues including liver, kidney, and uterus were analyzed for bisphenol A 24 h after the last treatment. Data represented mean and S.D. (n =4~ 5). VC; vehicle control (corn oil with 10% ethanol), BPL; bisphenol A 200 mg/kg, BPH; bisphenol A 400 mg/kg, PPL; propyl paraben 200 mg/kg, PPH; propyl paraben 400 mg/kg, ML; mixture of propyl paraben (200 mg/kg) and bisphenol A (200 mg/kg), MH; mixture of propyl paraben (400 mg/kg) and bisphenol A (400 mg/kg). 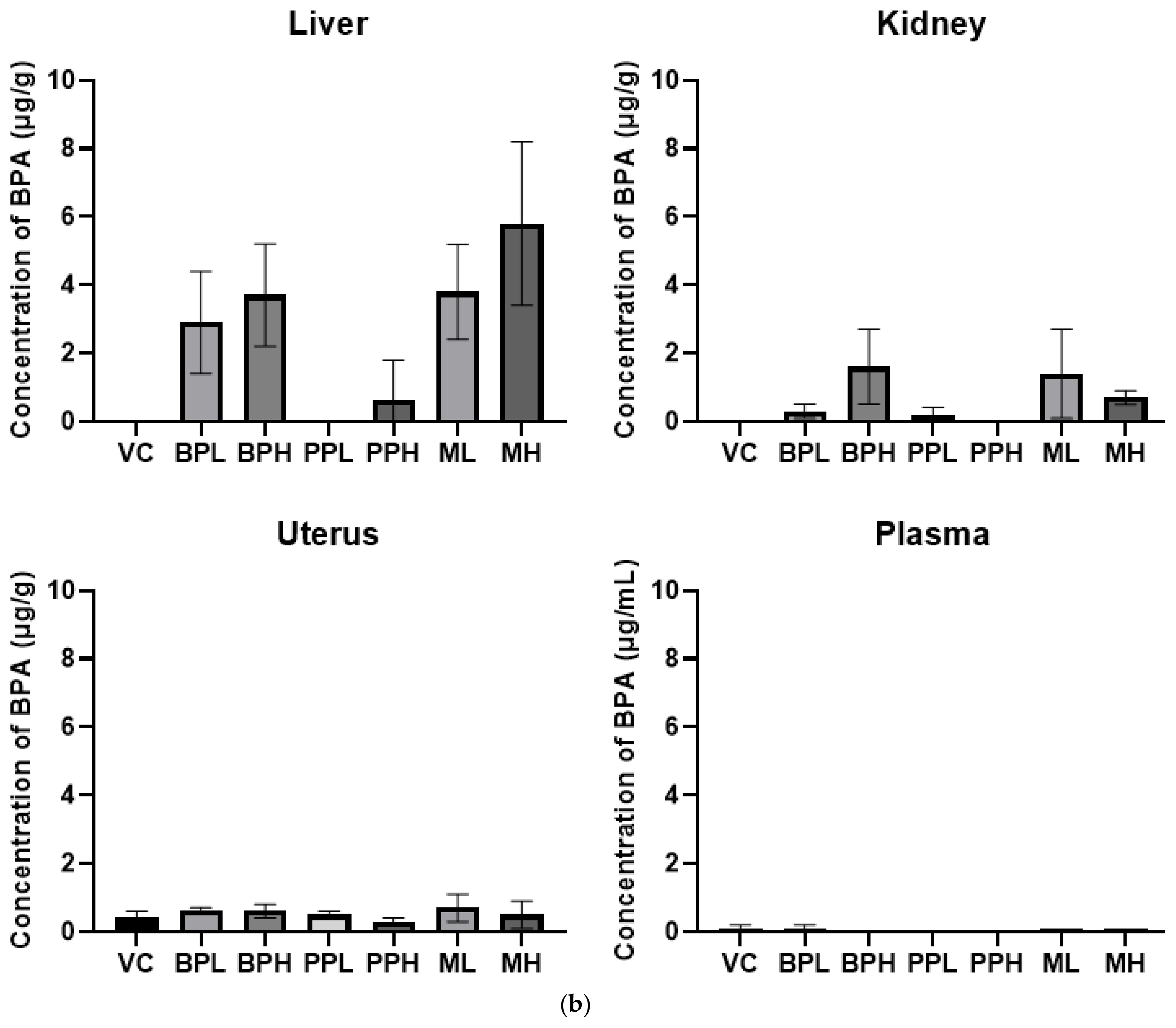
Table 1List of test substances, group names, and treated doses. VC; vehicle control (corn oil with 10% ethanol), PC; positive control (0.3 mg/kg 17β-estradiol), BPL; bisphenol A 200 mg/kg, BPH; bisphenol A 400 mg/kg, PPL; propyl paraben 200 mg/kg, PPH; propyl paraben 400 mg/kg, ML; mixture of propyl paraben (200 mg/kg) and bisphenol A (200 mg/kg), MH; mixture of propyl paraben (400 mg/kg) and bisphenol A (400 mg/kg). Table 2Absolute weights (g) and relative ratios (%) of the tissues. Results represented the mean ± standard deviation (SD). Statistical significance was considered at p < 0.05. Compared with the VC, the absolute and relative uterus weight in the PC group was significantly increased, respectively (p<0.05). Those of MH also increased compared to VC, but no statistical significance was observed. No changes in organ weight were observed in the liver and kidney. VC; vehicle control (corn oil with 10% ethanol), PC; positive control (0.3 mg/kg 17β-estradiol), BPL; bisphenol A 200 mg/kg, BPH; bisphenol A 400 mg/kg, PPL; propyl paraben 200 mg/kg, PPH; propyl paraben 400 mg/kg, ML; mixture of propyl paraben (200 mg/kg) and bisphenol A (200 mg/kg), MH; mixture of propyl paraben (400 mg/kg) and bisphenol A (400 mg/kg). Table 3The results of the uterus histopathologic findings in rats after 3 consecutive oral administrations.
Grade: +; mile, +++; marked. For the three viewpoints, significant changes were observed in PC and mild changes in MH were observed. VC; vehicle control (corn oil with 10% ethanol), PC; positive control (0.3 mg/kg 17β-estradiol), MH; mixture of propyl paraben (400 mg/kg) and bisphenol A (400 mg/kg). Table 4The results of the hematological analysis in rats after 3 consecutive oral administrations. Results represented the mean ± standard deviation (SD). Statistical significance was considered at p < 0.05. All the parameters were within the normal range, and there were no significant differences between the vehicle and chemical-treated groups. RBC; red blood cell count, HGB; hemoglobin, HCT; hematocrit, MCV; mean corpuscular volume, MCH; mean corpuscular hemoglobin, MCHC; mean corpuscular hemoglobin concentration, RDW; red cell distribution width, MPV; mean platelet volume, PLT; platelets, WBC; white blood cell, NEU; neutrophil, LYM; lymphocyte, MONO; monocyte, EOS; eosinophil, BASO; basophil. VC; vehicle control (corn oil with 10% ethanol), PC; positive control (0.3 mg/kg 17β-estradiol), BPL; bisphenol A 200 mg/kg, BPH; bisphenol A 400 mg/kg, PPL; propyl paraben 200 mg/kg, PPH; propyl paraben 400 mg/kg, ML; mixture of propyl paraben (200 mg/kg) and bisphenol A (200 mg/kg), MH; mixture of propyl paraben (400 mg/kg) and bisphenol A (400 mg/kg). Table 5The results of plasma biochemistry analysis in rats after 3 consecutive oral administrations. Results represented the mean ± standard deviation (SD). Statistical significance was considered at p < 0.05. Statistical differences between VC and treated groups were observed in some parameters, but they are in the normal range. The effect of the test chemical could not be confirmed. AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, BUN; blood urea nitrogen, TG; triglyceride, HDL-C; high-density lipoprotein cholesterol, LDL-C; low-density lipoprotein cholesterol, CK; creatine kinase. VC; vehicle control (corn oil with 10% ethanol), PC; positive control (0.3 mg/kg 17β-estradiol), BPL; bisphenol A 200 mg/kg, BPH; bisphenol A 400 mg/kg, PPL; propyl paraben 200 mg/kg, PPH; propyl paraben 400 mg/kg, ML; mixture of propyl paraben (200 mg/kg) and bisphenol A (200 mg/kg), MH; mixture of propyl paraben (400 mg/kg) and bisphenol A (400 mg/kg). |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||