Introduction
Heavy metals such as lead (Pb) and cadmium (Cd) exist as particulate matter (PM) in the air and can cause biological damage to cells, animals, and humans [1]. Heavy metals are major environmental neurotoxins that cause neuroinflammation through reactive oxygen species (ROS) production, leading to health risks such as Alzheimer’s disease, Parkinson’s disease, frontotemporal lobar degeneration, and amyotrophic lateral sclerosis [2, 3]. Pb toxicity is associated with the development of neurological diseases, hypertension, and cognitive impairment, causing brain toxicity and neuroinflammation [4]. The mechanisms underlying the neurotoxic effects of Pb have been linked to cytotoxicity, alterations to neurotransmitter storage and release, the induction of brain cell apoptosis, inflammation, and oxidative stress [5]. However, Cd exposure causes neurotoxicity in the hippocampus, resulting in cognitive impairment and psychomotor dysfunction [3]. Cd can also trigger oxidative stress in nerve tissue and affect endocrine functions [6]. The accumulation of these heavy metals due to excessive exposure results in neurodegeneration [7], revealing a toxicity mechanism similar to that of oxidative stress generation, a hallmark of degenerative neurological diseases [8].
As the main site of ROS generation, the mitochondria are prone to pathological changes associated with oxidative stress that have lethal consequences such as mitochondrial DNA (mDNA) damage, ultimately promoting apoptosis [9]. Upon mitochondrial dysfunction, endothelial cells secrete multiple pro-inflammatory cytokines, including IL-1, IL-6, and TNF-α [1]. Of these, IL-6 and IL-8 are well-known cytokines associated with cancer progression [1].
Interest in the toxicity mechanisms of heavy metals and factors contributing to various neurological diseases is increasing, with many studies being conducted to this end. However, results regarding the effects of heavy metals on neurological diseases differ between reports, and the mechanism underlying the neurotoxicity of heavy metals remains elusive. Glioma is the most common and fatal tumor of the central nervous system. According to the World Health Organization classification, gliomas can be divided into grades 1 to 4 based on the degree of malignancy [10]. U87 cells are a human glioblastoma cell line commonly used in research on brain cancer [11], including aggressive malignant gliomas, the most common and aggressive primary type of brain tumor [12]. According to a study by Yuan et al., and Liu et al., the critical toxic concentration (EC50) of PM detected as a result of Cd (1.00 × 10-3 mg/kg), Pb (3.52 × 10-3 mg/kg), and rReal-time cell analysis was 107.90 mg/L (r2 = 1.00, p < 0.01) [19, 20]. The calculated concentrations of Cd and Pb that correspond to the EC50 of PM are 379.8 μg/L and 101.9 μg/L, respectively. When two heavy metals are selected to describe the uniform concentration below this, Pb (1 μg/L, 30 μg/L, 1 mg/L) and Cd (1 μg/L, 30 μg/L, 1 mg/L).Therefore, the purpose of this study was to analyze the viability of U87 cells exposed to low concentrations of Cd and Pb and the effect on lactic acid dehydrogenase (LDH) activity and interleukin-6 (IL-6) concentrations to provide basic reference evidence of the effect of Cd and Pb on U87 cells and the mechanism of heavy metal toxicity.
Materials and Methods
Cell invasion assay
Cell culture
A human brain glioblastoma epithelial cell line (U87) was purchased from the Korean Cell Line Bank (Seoul, Republic of Korea) and cultured in minimum essential Eagle’s medium (M4655; Sigma Gangnam-gu, Republic of Korea) in a humidified atmosphere (5% CO2) at 37 °C. All media were supplemented with 10% fetal bovine serum (FBS; Corning, Glendale, CA, USA), 100 units/mL penicillin, and 100 units/mL streptomycin. Cells (1 × 104 cells/well) were seeded in 96-well plates and incubated at 37 °C with 5% CO2 for 24 h.
Chemicals
Cd standard solution-1000 ppm (Cat. No. 07993-1B) and Pb standard solution-1000 ppm (Cat. No. 24239-2B) were obtained from Kanto Chemical (Tokyo, Japan). Cd and Pb were diluted in Dulbecco′s phosphate-buffered saline solution (Welgene, Gyeongsan-si, Republic of Korea). Cells were treated with Cd and Pb at concentrations of 1 μg/L, 30 μg/L, and 1 mg/L for 0 and 24 h.
Water-soluble tetrazolium (WST) assay for cell viability
Cell viability
The effect of Cd and Pb on cell viability was determined using the Quanti-MAX WST-8 (tetrazolium salt) Cell Viability Assay Kit (Biomax, Seoul, Republic of Korea). Briefly, 10 μL of WST was added to each well and incubated at 37 °C for 0.5 h. The absorbance was measured at 450 nm using a Flex Station 3 Multi-Mode Microplate Reader (Molecular Devices, San Jose, USA). RPMI medium without Cd and Pb was used as a negative control. Changes in cell viability were determined in triplicate. Subsequently, cells (1 × 104 cells/well) were seeded in 96-well plates and incubated at 37 °C with 5% CO2 for 24 h. The cell viability of the Cd- and Pb-exposed cells was expressed as a percentage relative to that of non-exposed cells.
LDH release
LDH release from the cells into the culture medium was monitored using the Quanti-LDH PLUS Cytotoxicity Assay Kit (Biomax). The cytotoxicity of Cd and Pb was measured by determining the LDH concentration using a colorimetric assay. Aliquots (100 μL) of the cell culture medium were collected from each well and placed into microtiter plates. LDH (100 μL) was added to each well, and the plates were incubated at 37 °C with 5% CO2 for 0.5 h; then, the absorbance of the samples was measured at 490 nm using a Flex Station 3 Multi-Mode Microplate Reader (Molecular Devices, CA, USA). Each experiment was performed in quadruplicate. The cytotoxicity was relative to the basal LDH of the untreated control cells and medium without FBS. The test was replicated three times, and the amount of LDH released by the Cd- and Pb-exposed cells was expressed as a percentage relative to that released by non-exposed cells.
IL-6
IL-6 levels were monitored using the Human IL-6 ELISA MAX™ Deluxe Set (BioLegend, CA, USA). One day before conducting the ELISA procedure, the capture antibody was diluted in 1 coating buffer A, and then, 100 μL of this solution was added to all 96-well plates. The plate was then sealed and incubated overnight (16–18 h) at between 2 °C and 8 °C. Next, 100 μL of diluted avidin-HRP solution (BioLegend, CA, USA) and 100 μL of TMB substrate solution (BioLegend, CA, USA) were added. After incubating the samples for 15 min, the absorbance was measured at 490 nm using a FlexStation 3 multi-mode microplate reader (Molecular Devices, CA, USA).
Statistical analysis
Statistical analyses were performed using a one-way analysis of variance with Tukey’s test with SPSS. Results are expressed as the mean ± standard error of the mean. The results of LDH activity and IL-6 concentrations in U87 cells were analyzed according to the concentration and exposure time of Cd and Pb. Statistical significance was set at P < 0.05.
Results
U87 cell viability
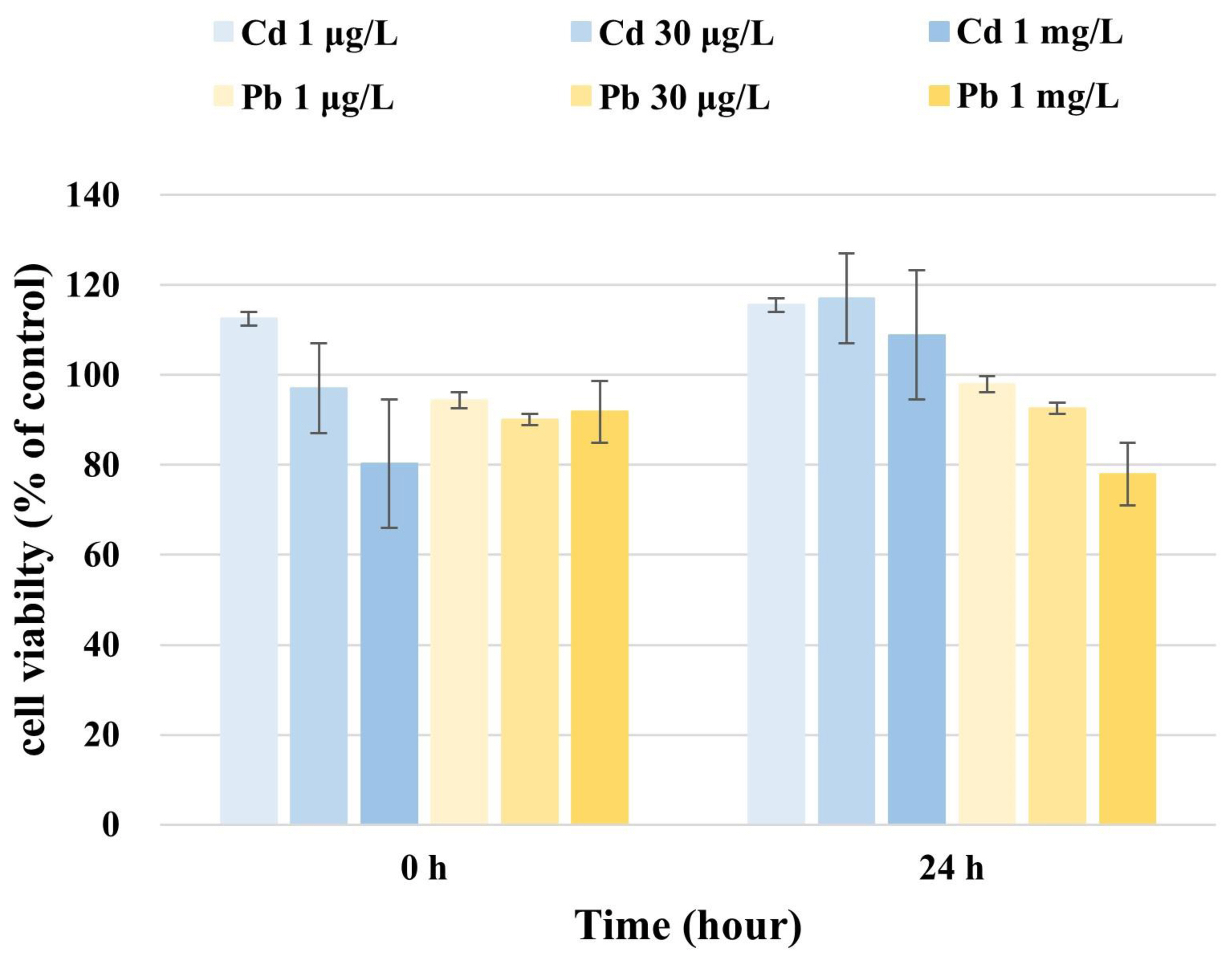
We calculated the WST-based cell viability by comparing the absorption of the culture medium of Cd-exposed U87 cells with that of unexposed cells. The average WST concentration at 0 h after adding 1 μg/L Cd was 112.5 μg/L in U87 cells, whereas that at 24 h was 115.6 μg/L (Fig. 1). After adding 30 μg/L Cd to U87 cells, the WST concentration measured at 0 h was 97.0 μg/L and was 117.0 μg/L after 24 h (Fig. 1). The average WST concentrations after adding 1 mg/L Cd to U87 cells were 80.3 μg/L and 108.9 μg/L after 0 h and 24 h, respectively (Fig. 1). These results indicate that the WST-based viability of U87 cells increased over time with concentrations of 1 μg/L, 30 μg/L, and 1 mg/L Cd.
We then calculated the WST-based cell viability by comparing the absorption of the culture medium of Pb-exposed U87 cells with that of unexposed cells. The average WST concentrations in U87 cells measured at 0 h and 24 h were 94.4 μg/L and 97.9 μg/L (1 μg/L Pb), 90.1 μg/L and 92.6 μg/L (30 μg/L Pb), and 91.8 μg/L and 77.9 μg/L (1 mg/L Pb), respectively (Fig. 1), indicating that the WST-based viability of U87 cells was reduced over time at a concentration of 1 mg/L Pb.
LDH activity in U87 cells
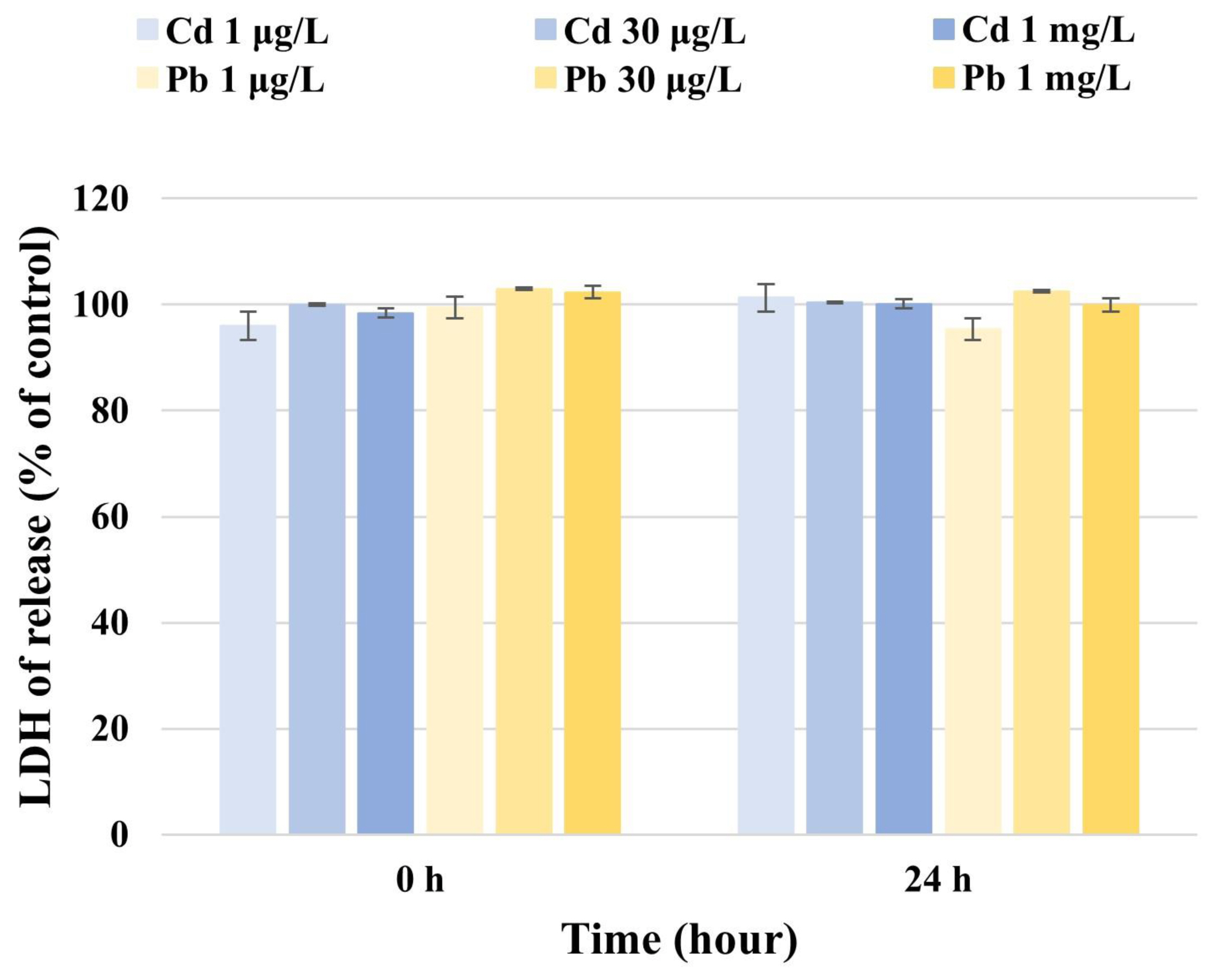
The LDH levels released from U87 cells were calculated by comparing the absorption of the medium of Cd-exposed cells with that of the control. The LDH activity measured in U87 cells at 0 h and 24 h was 96.0 μg/L and 101.3 μg/L (1 μg/L Cd), 100.0 μg/L and 100.3 μg/L (30 μg/L Cd), and 98.4 μg/L and 100.1 μg/L (1 mg/L Cd), respectively (Fig. 2). At Cd concentrations of 1 μg/L, 30 μg/L, and 1 mg/L, no difference in LDH activity was observed in U87 cells. These results suggest that Cd has no effect on LDH activity at the concentrations used in this study.
LDH levels released from U87 cells were calculated by comparing the absorption of the medium of Pb-exposed cells with that of the control. The LDH activity measured in U87 cells at 0 h and 24 h was 99.4 μg/L and 95.3 μg/L (1 μg/L Pb), 102.9 μg/L and 102.5 μg/L (30 μg/L Pb), and 102.3 μg/L and 99.9 μg/L (1 mg/L Pb), respectively (Fig. 2). At Pb concentrations of 1 μg/L, 30 μg/L, and 1 mg/L, no difference in LDH activity was observed in U87 cells. These results show that Pb has no effect on LDH activity at the concentrations used in this study.
IL-6 concentrations in U87 cells
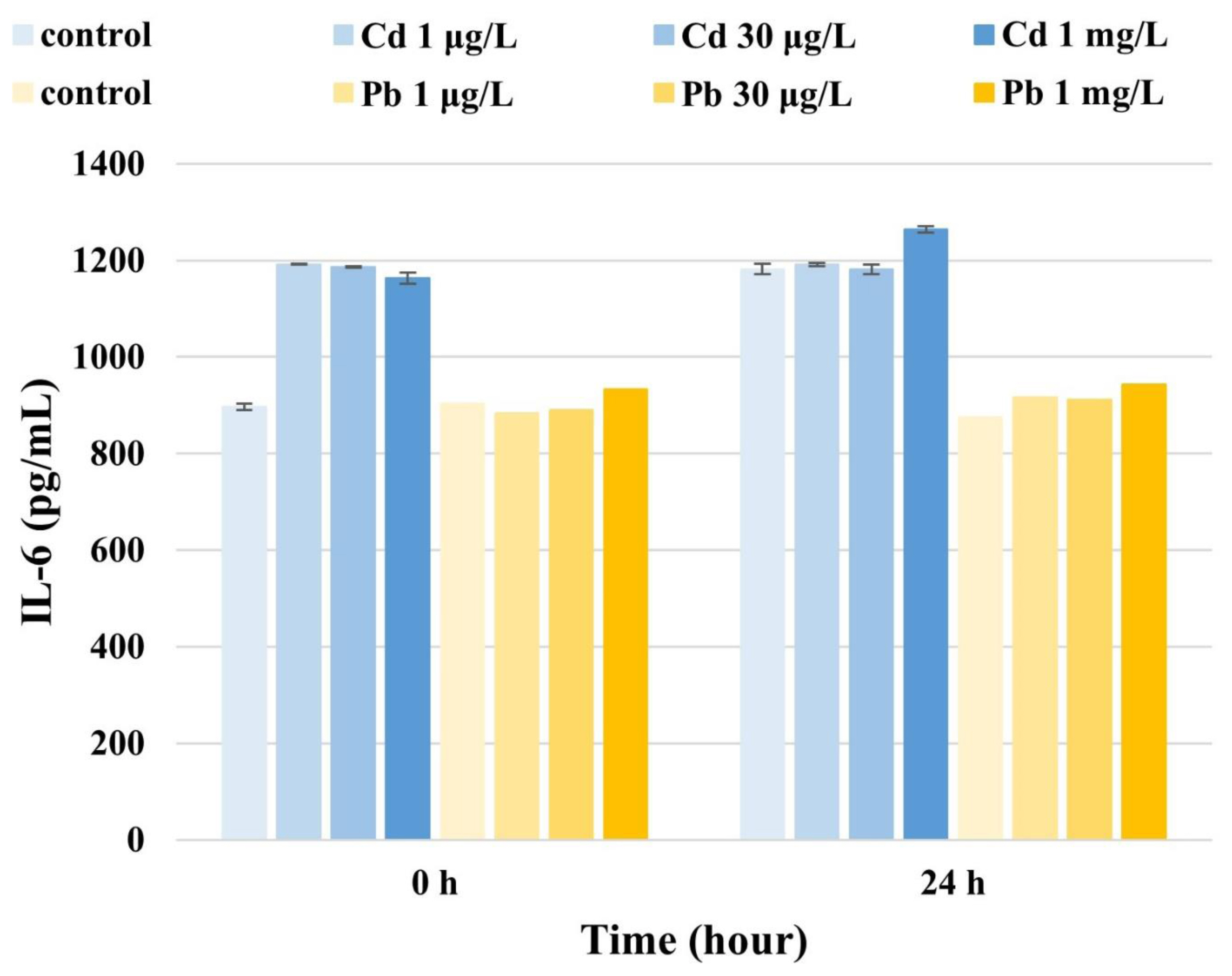
IL-6 levels in U87 cells measured at 0 h and 24 h were 1191.9 μg/L and 1190.9 μg/L (1 μg/L Cd), 1185.5 μg/L and 1181.7 μg/L (30 μg/L Cd), and 1163.0 μg/L and 1264.6 μg/L (1 mg/L Cd), respectively (Fig. 3). These results indicate that the IL-6 levels in U87 cells increased over time at concentrations of 10 μg/L, 30 μg/L, and 1 mg/L Cd. Together, these results suggest that IL-6 levels in U87 cells also increase over time upon exposure to high Cd concentrations..
IL-6 levels in U87 cells at 0 h and 24 h were 882.8 μg/L and 916.4 μg/L (1 μg/L Pb), 889.3 μg/L and 912.0 μg/L (30 μg/L Pb), and 932.8 μg/L and 942.6 μg/L (1 mg/L Pb), respectively (Fig. 3), showing that Pb dose-dependently affects IL-6 levels in U87 cells over time.
Discussion
In neuronal cells, Cd induces oxidative stress, which causes protein damage and neurodegeneration [13]. Further, Cd-treated nerve cells show marked changes in morphology and an increase in necrotic cells, eventually causing apoptosis [14]. In cell culture experiments, astrocytes are more resistant to acute high-doses of Cd (300 μM for 30 min) than neurons but more sensitive than neurons to long-term low-doses (10 μM) [15]. Significant decreases in cell viability have been observed with low micromolar Cd concentrations [16]. In contrast, the results of this study indicated that the WST-based viability of U87 cells increased after 24 h upon exposure to concentrations of 1 μg/L, 30 μg/L, and 1 mg/L Cd. Therefore, remarkable increases in cell viability were observed with low micromolar Cd concentrations.
The mechanisms underlying the neurotoxic effects of Pb have been linked to cytotoxicity, alterations to neurotransmitter storage and release, the induction of brain cell apoptosis, inflammation, and oxidative stress [5]. Pb2+-induced neurotoxicity can also lead to oxidative stress, inflammatory cytokine production, and neurocytosis [2]. Pb at micromolar concentrations exerts neurotoxic effects by inducing oxidative stress and inflammation, resulting in apoptosis [17]. Our results showed that the WST-based cell viability of U87 cells decreased after 24 h with 1 mg/L Pb concentrations.
Cd exposure increases the protein concentrations of IL-8, chemokine (C-C motif) ligand-26, IL-6, and IL-1 receptors, as well as ligand-1 receptors, owing to the reduced production of anti-inflammatory cytokines [18]. Our results also showed increased IL-6 levels in U87 cells after 24 h as the Cd concentration was increased. Lead exposure is associated with increased levels of inflammatory mediators (IgE, IL-1β, IL-4, IL-5, IL-6, IL-10, IL-13, IL-18, IL-33, and TNFα) in humans [5]. Our results showed that IL-6 levels in U87 cells also increased over time upon exposure to high Pb concentrations.
LDH is a biomarker reflecting cell membrane integrity; any damage to the membrane triggers the cells to release LDH [17]. Cd at low concentrations did not affect LDH activity in U87 cells throughout the experimental time used in this study. In contrast, an increase in LDH release in the presence of AP and/or Pb2+ could be attributed to their cytotoxic effects on these cells [17]. However, our results showed that Pb did not affect LDH activity in U87 cells.
This study had some limitations. First, our analysis was limited to Pb and Cd. Since several heavy metals were not included in the analysis, our results cannot be generalized to reveal the effect of heavy metals on nerve cells, possibly leading to biased interpretations. To more comprehensively investigate the fundamental effect of heavy metals on nerve cells, future studies, including those with additional heavy metals, are warranted. Second, in this study, the changes in U87 cells 24 h after exposure to Cd and Pb were analyzed. This is a relatively short-term response analysis, which could have influenced the effect of Cd and Pb on U87 cells, depending on the reaction time. Thus, our results might be insufficient to offer a conclusive interpretation of the effects of heavy metal exposure on nerve cell physiology.
Nonetheless, in this study, the viability of U87 cells exposed to low levels of Cd and Pb was assessed, revealing no significant effect on cell viability at low concentrations of both heavy metals. However, beyond cell viability, the IL-6 levels in U87 cells increased over time with high Cd and Pb concentrations, indicating their remarkable effect on the inflammatory responses of these cells. Our study confirmed that the viability of and IL-6 production by U87 cells vary with heavy metal concentration and exposure time. Therefore, the study results suggest that even low concentrations of heavy metals can promote inflammatory reactions in nerve cells. Thus, our findings provide the basis for future research elucidating the effects of heavy metals on cellular pathophysiology. Systematic studies with higher heavy metal concentrations and precision are needed to further understand the relationship between heavy metals and neuronal physiology.