AbstractOrganophosphorus insecticides such as diazinon (DZN) are used worldwide in industry, veterinary practice, and agriculture. They may induce oxidative stress in different tissues. The use of antioxidants can protect tissues against oxidative stress. The aim of this study was to investigate the prophylactic and therapeutic roles of vitamin E against DZN–induced oxidative damage and biochemical alterations in various tissues of male Wistar rats. Thirty rats were divided into five groups: Control group received only corn oil as DZN solvent, DZN group received 100 mg/kg of DZN, E group received 150 mg/kg of vitamin E, E-DZN group received vitamin E and then dosed with DZN and DZN-E group received DZN and then dosed with vitamin E. All injections were carried out intraperitoneally. Plasma and various tissues were prepared and evaluated. Results showed that acute administration of DZN caused a significant induction of oxidative damage in the tested tissues via increased malondialdehyde level and some plasma biochemical indices, depletion of glutathione (GSH), reduced cholinesterase activity and change in the activities of superoxide dismutase, catalase and glutathione-S transferase. Treatment of rats with vitamin E resulted in an elevation in the level of GSH, normalizing the antioxidant enzymes activities and decreasing lipid peroxidation, although all these tests did not return to the normal level in certain tissues. The findings of this study suggest that both prophylactic and therapeutic treatments of rats with vitamin E provide a protective role against DZN-induced oxidative stress and cholinergic hyperactivity through free radicals scavenging and membrane stabilizing.
IntroductionOrganophosphates (OP) are a bunch of pesticides that are widely used for a variety of agricultural, medical, and industrial applications. These compounds inhibit acetylcholine-esterase (AChE) enzyme activity, which causes the accumulation of acetylcholine (ACh) in the synaptic cleft that causes hypercholinergic symptoms. Poisoning by OPs is one of the global health problems and are responsible for the third most common cause of poisoning in Iran [1–3]. Diazinon (DZN) is one of the most important OP insecticides that is used extensively for various health-related and agricultural purposes pesticides in Iran [4]. Its residues in foods, water, vegetables and soil have direct effects on health of humans and animals. DZN is a highly toxic OP pesticide that oxidatively bioactivates to diazoxon by the liver microsomal enzymes system [3,5]. DZN causes some hematological, biochemical and histological alterations in rat, rabbits and mice. Its exposure may also cause alterations many systems such as the immune system, urinary system, reproductive system, pancreas and liver [2,6]. Furthermore, DZN produces oxidative stress through the formation of reactive oxygen species (ROS) and alterations in antioxidants status in mammals and other organisms [2,7,8].
Antioxidants constitute the primary defense system that limits the toxicity associated with ROS. DZN is a lipophilic compound with a long half-life that binds extensively to biological membranes and enhances lipid peroxidation [9]. Vitamin E (α-tocopherol) as the most important lipophilic antioxidant is a natural component of the membrane lipid bilayer of cells and thus preserving membrane integrity. It inhibits free radical formation and may effectively minimize lipid peroxidation resulting oxidative stress in biological systems because of its oxygen scavenging effect [8,10]. Several studies have been investigated on the effect of chronic administration of DZN and vitamin E for 14 and 21 days on biochemical parameters and/or antioxidant defense status in rat liver and erythrocytes [11], mice liver [12] and rat heart [13]. However, few studies have been performed on acute exposure of DZN and combination of vitamin C and vitamin E [14,15]. To the best of our knowledge, there is no report on the prophylactic and therapeutic roles of vitamin E on DZN-induced oxidative stress in vital tissues after intraperitoneal injection. In the present study, we tried to look at these effects with assessing the activities of free radical scavenging enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), as well as malondialdehyde (MDA) level as an important index of lipid peroxidation and glutathione (GSH) concentration in liver, erythrocytes, kidney, brain, heart and spleen and biochemical parameters measurement in plasma of male Wistar rats.
Materials and MethodsChemicalsDZN, purity 99% was obtained from Supelco Company (USA). All chemicals were of the highest analytical grade from Sigma and Merck. DZN and vitamin E dissolved in corn oil at a stock concentration of 600 mg/mL, immediately before use.
AnimalsMale Wistar rats weighing 170–230 g were purchased from Baqiyatallah University of Medical Sciences (Tehran, Iran) and kept for 1 week before the beginning of the experiment for acclimatization. Animals were housed three to a cage at room temperature (25±2 °C) with a relative humidity of 50–60% and on a 12 h light–darkness cycle. The animals had free access to commercial pellet diet and water ad libitum. The ethics committee of the Baqiyatallah University of Medical Sciences approved the experimental protocol, and all efforts were made to minimize the animal suffering.
Experimental designThe animals were randomly divided into five groups having six rats in each group. Control group was treated with corn oil as DZN solvent. DZN group was treated with 100 mg/kg of DZN [7]. E group was treated with 150 mg/kg of vitamin E [8]. Rats in E-DZN group were pre-treated with 150 mg/kg of vitamin E and then dosed with DZN (100 mg/kg), 30 min later. Rats in DZN-E group were pre-treated with 100 mg/kg of DZN and then dosed with vitamin E (150 mg/kg), 30 min later. All injections were carried out intraperitoneally. All rats were weighed at the beginning and at the end of the study.
Plasma and tissues preparationRats in each group were sacrificed 24 hours [7,16] after the last administration following overnight fast. Blood samples were collected by cardiac puncture into heparinized test tubes, and immediately centrifuged at 3,000×g for 15 min at 4 °C. Plasma was removed and erythrocytes were washed three times with five volumes of phosphate-buffered saline (PBS), centrifuged as above, and after removal of the supernatant fluid and the white buffy layer, were divided into equal portions and frozen at −70 °C until use. Also, liver, kidney, brain, heart, and spleen were quickly removed, washed in ice-cold PBS. Washed tissues were immediately immersed in liquid nitrogen and stored at −70 °C until biochemical analysis.
On the day of use, erythrocytes were hemolyzed in 10 volumes of ice-cold distilled water. After centrifugation, the supernatant was used for biochemical analysis. In addition, frozen tissue samples were quickly weighed and homogenized 1:10 in ice-cold PBS in a Heidolph type homogenizer (Germany). The homogenates were then centrifuged at 16,000×g for 15 min at 4 °C. The supernatants were separated and used for enzyme activities assays and determination of GSH, MDA and protein levels.
Plasma biochemical parameters assaysActivities of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH) and creatin phosphate kinase (CK), levels of urea, creatinine and uric acid were determined in plasma using Parsazmun Company kits (Tehran-Iran).
ChE activity assayChE activity in cell lysates was determined at 412 nm by a slight modification of Ellman method [17]. The reaction mixture containing 0.05 mL homogenate and 0.423 mM 5, 5′-dithiobis 2-nitrobenzoic acid (DTNB) in 0.1 M sodium phosphate buffer, pH 7.5 was incubated at 37 °C for 5 min. Reaction was initiated by addition of 20 mM acetylthiocholine iodide as the substrate for the erythrocytes, brain and spleen cells and butyrylthiocholine iodide for the liver, kidney and heart. The rate of increase of absorbance was measured on a Genesys 10 UV spectrophotometer at 412 nm during 5 min. The enzyme activity is expressed as U/ mg protein.
Biomarkers of oxidative damageThe activity of SOD was determined based on the ability of SOD to inhibit the reduction of nitroblue tetrazolium by superoxide. CAT activity was measured at 240 nm by calculating the rate of degradation of hydrogen peroxide (H2O2) as the substrate of the enzyme. GST activity was assayed by monitoring the formation of the thioether product of the reaction between GSH and 1-chloro-2, 4-dinitrobenzene at 340 nm. GSH level was assayed by monitoring the absorbance of DTNB at 412 nm. MDA level as an indicator of lipid peroxidation was determined at 532 nm using 2-thiobarbituric acid [7,18].
Protein and hemoglobin levelsThe total protein concentration in tissues was measured by Bradford’s method [19]. Appropriate volume of sample reached the volume of 1 mL to which 3 mL of Bradford solution was added and was incubated for 10 minutes. Then, absorbance was read at 595 nm. Protein concentration was calculated using 1mg/mL bovine serum albumin solution as standard.
Hemoglobin (Hb) concentration in blood was measured by Van Kampen method [20]. 100 μL of whole blood samples were mixed with 5 mL of Drabkin’s solution and the hemoglobin concentration was measured with a hemoglobinometer (ERMA INC, Japan). Protein and hemoglobin concentrations were used to normalize enzyme activities and levels of GSH and MDA.
Statistical analysisStatistical analysis of the data was conducted using SPSS statistical software version 22 (IBM Corporation, USA). Significance was determined by analysis of variance (ANOVA) followed by post hoc analysis using Tukey multiple comparison tests applied across treatment groups for each tissue. Analysis of the correlation between oxidative stress biomarkers and ChE activity was performed using Pearson correlation analysis. Results were expressed as mean±SD. Significance level was based on p<0.05.
Results and DiscussionEffects of treatments on plasma biochemical parametersThe increase in the activities of ALT, AST, LDH, ALP and CK enzymes and urea, uric acid and creatinine levels are used as important diagnostic tool for cellular damage and cytotoxicity of toxic agents [21–23]. In this study, DZN significantly increased plasma AST, ALT, ALP, CK and LDH activities and urea, uric acid and creatinine levels compared with those of the control group (p<0.05) (Figure 1 and 2). The elevated these enzymes activities in DZN exposed rat may be due to leakage from the liver and heart tissues as a result of DZN-induced lipid peroxidation of membrane [24]. In addition, significant increases of plasma creatinine, urea and uric acid levels may due to the impairment of the glomerular function and tubular damage in the kidneys caused by DZN through induction of oxidative stress [2,5]. Similarly, ROS produced from the metabolism of DZN may damage other tissues such as heart, kidney and spleen causing the leakage of enzymes from the tissues into plasma [21]. However, the reduced these parameters levels in groups pre- and post-treated with vitamin E showed that the antioxidant vitamin E protected the tissues from the lipoperoxidative changes provoked by DZN, probably due to its free radical scavenging ability [2,25]. Damodar et al. [26] have reported that co-administration of vitamin E with DZN to rats resulted in insignificant improvement of the liver enzyme activities. Altuntas and Delibas [27] observed that vitamin E did not show effect on some biochemical indices.
Effects of treatments on ChE and antioxidant enzymes activitiesThe most important action of DZN compound toxicity is the inhibition of ChE activity and the accumulation of ACh in the peripheral and central nervous system leading to increased activation of nicotinic and muscarinic receptors [28]. In the present study, in vivo administration of DZN inhibited ChE activity in all tissues of rat and administration of vitamin E in the protective and therapeutic groups alleviated the DZN toxicity and maintained the activity of ChE near to the normal value of control in the liver, kidney, brain and spleen (Table 1). This may be due to increase the activity of paraoxonase by vitamin E [29], which is essential in the detoxification of OP and also aids in the reactivation of AChE [30]. Similar results were shown by previous studies [5,31]. A study showed that after 4 weeks of DZN administration, a reduction in plasma ChE activity was observed and vitamin E did not affect the ChE activity [32].
Oxidative stress occurs in the tissues by DZN exposure through increased production of ROS and/or decreased capacity of antioxidant defense. The antioxidant enzymes including SOD and CAT protect cells against the deleterious effects of oxidative stress by converting free radicals to non-radical products. SOD converts superoxide anion radicals into H2O2 and O2. Then, H2O2 is converted into H2O and O2 by CAT [7,33,34]. GST catalyses conjugation of GSH with DZN or ROS and produces more water soluble and excreteable compounds. Therefore, it can decrease the toxicity of DZN and protects tissues from lipid peroxidation and oxidative stress [10]. In this study, DZN increased SOD and GST activities in all tissues and CAT activity in liver, kidney and heart. It also decreased CAT activity in erythrocytes, brain and spleen. There were significant differences in SOD and CAT activities in brain between DZN group and E-DZN group (Table 1). The increased of these antioxidant enzymes activities are probably a response to neutralize the impact of increased ROS generation in tissues [34]. The depletion of CAT activity may be a consequence of irreversible inactivation of enzyme proteins from increased ROS production resulting from DZN metabolism. The increased GST activity was associated with an increase in GSH consumption (Figure 3) indicates the increase of body’s defense against DZN, and rapid excretion of it [35]. However, pre and post-administration of vitamin E had the ability to recover these parameters due toits ability to scavenge the accumulated free radicals leading to limiting the effects of ROS on the tissues, and thus it protects the cell from injury [11,36,37]. Vitamin E is a nonenzymatic antioxidant in the lipid phase of cells, acts to neutralize the toxic effects of ROS, which are potentially damaging by-products of the body’s metabolism [8]. Previous studies performed in animals support our study findings. Ibrahim [37] reported that SOD and CAT activities were significantly increased in kidney and liver tissues of fish after 14 and 28 days exposure to DZN, while these enzymes were significantly decreased in DZN-exposed fishes fed with diets supplemented with vitamin E. Akturk et al. [14] showed that DZN caused significant increases in the activities of SOD and CAT in the kidney of rat and the treatment with a combination of vitamins E and C 30 min after the administration of DZN was somewhat effective in restoring the activities of these enzymes. Messarah et al. [11] indicated that CAT activity was significantly decreased in rat liver and erythrocytes after DZN orally administration for 21 days and the supplementation of vitamin E significantly modulated the activity of this enzyme. El-Shenawy et al. [12] showed a significant decrease in SOD and CAT activities in liver by DZN-exposed mice for 14 days and these effects were largely prevented by a vitamin E supplementation. However, Yilmaz et al. [8] found that only the activity of SOD was directly mediated by DZN exposure and co-treatment with vitamins E and C.
Effects of treatments on GSH levelGSH plays a crucial role in intracellular protection against ROS. It is involved in maintenance of other antioxidants, such as ascorbate and α-tocopherol. GSH is also required for the antioxidant enzymes activities such as glutathione peroxidase and GST [7,38]. In the current study, GSH level was significantly decreased in all tissues of DZN-treated rat comparing to the control (Figure 3). The decreased tissues GSH content indicates impair the cells defense against the toxic actions of ROS, which may lead to oxidative stress and cytotoxicity [2]. However, administration of vitamin E with DZN in the prophylactic and therapeutic groups were returned GSH level near to the control levels, which can be directly correlated to the scavenging of oxidative free radicals. Our previous studies had shown that simultaneous treatment of vitamin E to the DZN-exposed rats was somewhat effective in restoring the activity of GST and level of GSH in different tissues [10,39–43].
Effects of treatments on MDA levelMDA is a stable metabolite of the free radical-mediated lipid peroxidation cascade and it is known as a marker of oxidative stress [22,44]. Our results showed a significant increase in the level of MDA in all tissues except spleen (Figure 4), which might have resulted from oxidative damage induced by DZN, might be due to its lipophilicity, whereby it could penetrate easily to the cell membrane and caused membrane lipid peroxidation [9]. However, pre and post-administration of vitamin E resulted in a significant reduction in lipid peroxidation in DZN treated rats due to the potency of vitamin E to scavenge the free radicals, which are generated during the oxidation of unsaturated lipids and lead to the propagation of lipid peroxidation [12]. Vitamin E allow free radicals to attract a hydrogen atom from the antioxidant molecule rather than from polyunsaturated fatty acids, thus breaking the chain of free radical reactions, the resulting antioxidant radical being a relatively non-reactive species [45]. In several studies, chronic administration of DZN with vitamin E only or in combination with vitamin C significantly decreased MDA level in various tissues [8,11,12,37].
Analysis of correlation between brain oxidative stress biomarkers and ChE activityDue to changes in oxidative stress biomarkers in tissues, in the present study, the toxicity of DZN in brain tissue is higher than other tissues. High ratio of membrane surface area of brain to cytoplasmic volume, high content of polyunsaturated fatty acids and the presence of Fe and oxygen, contribute to the accumulation of lipid peroxidation products during oxidative stress induced by DZN products [2,21]. AChE activity in brain was positively correlated with CAT activity and GSH level and negatively correlated with SOD activity and MDA level (Figure 5), suggesting that increased oxidative stress and lipid peroxidation by DZN may be a result of cholinergic hyperactivity which may be an initiator of accumulation of free radicals and cell injury [2]. By comparing the effects of vitamin E on oxidative stress biomarkers in various tissues, it seems that the effects of the restoring in the brain are higher than other tissues.
ConclusionsThe findings of the present study suggest that oxidative stress may be involved in DZN toxicity. However, both prophylactic and therapeutic treatments of rats with vitamin E is able to ameliorate the acute toxic effect of DZN on some biochemical and oxidative stress indices through inhibiting the ROS and lipid peroxidation generation, preventing the decline of antioxidant defense system and scavenging free radicals (Figure 6).
AcknowledgementThe authors would like to thank H. Mahdavi and J. Rasouli for their assistance. This work was supported by Chemical Injuries Research Center of Baqiyatallah University of Medical Sciences.
Conflict of interestConflict of interest
The authors declare that they have no conflict of interest.
NotesCRediT author statement
MJ: Project administration, Formal analysis, Conceptualization, Writing-Reviewing & Editing; KT: Resources, Visualization, Investigation; JH: Resources, Visualization, Investigation; MSA: Resources, Visualization, Investigation; AA: Supervision, Conceptualization, Methodology, Writing-Reviewing & Editing; MS: Formal analysis, Software; SK: Formal analysis, Software.
References1. Jalili C, Farzaei MH, Roshankhah S, Salahshoor MR. Resveratrol attenuates malathion-induced liver damage by reducing oxidative stress. J Lab Physicians 2019;11(03):212-219
https://doi.org/10.4103/JLP.JLP_43_19
.
2. Khazaie S, Jafari M, Heydari J, Asgari A, Tahmasebi K, Salehi M, et al. Modulatory effects of vitamin C on biochemical and oxidative changes induced by acute exposure to diazinon in rat various tissues: Prophylactic and therapeutic roles. J Anim Physiol Anim Nutr 2019;103(5):1619-1628
https://doi.org/10.1111/jpn.13144
.
3. Estakhri MA, Shokrzadeh M, Jaafari MR, Karami M, Mohammadi H. Organ toxicity attenuation by nanomicelles containing curcuminoids: Comparing the protective effects on tissues oxidative damage induced by diazinon. Iran J Basic Med Sci 2019;22(1):17-24
https://doi.org/10.22038/ijbms.2018.23229.5874
.
4. Pirsaheb M, Fattahi N, Rahimi R, Sharafi K, Ghaffari HR. Evaluation of abamectin, diazinon and chlorpyrifos pesticide residues in apple product of Mahabad region gardens: Iran in 2014. Food Chem 2017;231: 148-155
https://doi.org/10.1016/j.foodchem.2017.03.120
.
5. Mansour SA, Abbassy MA, Shaldam HA. Hepato-renal toxicity induced by chlorpyrifos, diazinon and their mixture to male rats with special concern to the effect of zinc supplementation. J Toxicol Pharmacol 2017;1(3):15-24.
6. Yassa VF, Girgis SM, Abumourad IM. Potential protective effects of vitamin E on diazinon-induced DNA damage and some haematological and biochemical alterations in rats. J Mediter Ecol 2011;11: 31-39.
7. Jafari M, Salehi M, Ahmadi S, Asgari A, Abasnezhad M, Hajigholamali M. The role of oxidative stress in diazinon-induced tissues toxicity in Wistar and Norway rats. Toxicol Mech Methods 2012;22(8):638-647
https://doi.org/10.3109/15376516.2012.716090
.
8. Yilmaz N, Yilmaz M, Altuntas I. Diazinon-induced brain toxicity and protection by vitamins E plus C. Toxicol Ind Health 2012;28(1):51-57
https://doi.org/10.1177/0748233711404035
.
9. Karami-Mohajeri S, Ahmadipour A, Rahimi HR, Abdollahi M. Adverse effects of organophosphorus pesticides on the liver: a brief summary of four decades of research. Arh Hig Rada Toksikol 2017;68(4):261-275
https://doi.org/10.1515/aiht-2017-68-2989
.
10. Tahmasebi K, Jafari M, Ahmadi A. Evaluation of oxidative stress biomarkers in rat heart exposed to diazinon and vitamins E and C. Horizon Med Sci 2015;21(1):13-19
https://doi.org/10.18869/acadpub.hms.21.1.13
.
11. Messarah M, Amamra W, Boumendjel A, Barkat L, Bouasla I, Abdennour C, et al. Ameliorating effects of curcumin and vitamin E on diazinon-induced oxidative damage in rat liver and erythrocytes. Toxicol Ind Health 2013;29(1):77-88
https://doi.org/10.1177/0748233712446726
.
12. El-Shenawy NS, El-Salmy F, Al-Eisa RA, El-Ahmary B. Amelioratory effect of vitamin E on organophosphorus insecticide diazinon-induced oxidative stress in mice liver. Pestic Biochem Physiol 2010;96(2):101-107
https://doi.org/10.1016/j.pestbp.2009.09.008
.
13. Ogutcu A, Uzunhisarcikli M, Kalender S, Durak D, Bayrakdar F, Kalender Y. The effects of organophosphate insecticide diazinon on malondialdehyde levels and myocardial cells in rat heart tissue and protective role of vitamin E. Pestic Biochem Physiol 2006;86(2):93-98
https://doi.org/10.1016/j.pestbp.2006.01.010
.
14. Akturk O, Demirin H, Sutcu R, Yilmaz N, Koylu H, Altuntas I. The effects of diazinon on lipid peroxidation and antioxidant enzymes in rat heart and ameliorating role of vitamin E and vitamin C. Cell Biol Toxicol 2006;22(6):455-461
https://doi.org/10.1007/s10565-006-0138-5
.
15. Gokalp O, Buyukvanlı B, Cicek E, Ozer MK, Koyu A, Altuntas I, et al. The effects of diazinon on pancreatic damage and ameliorating role of vitamin E and vitamin C. Pestic Biochem Physiol 2005;81(2):123-128
https://doi.org/10.1016/j.pestbp.2004.11.001
.
16. Ajibade TO, Oyagbemi AA, Omobowale TO, Asenuga ER, Afolabi JM, Adedapo AA. Mitigation of diazinon-induced cardiovascular and renal dysfunction by gallic acid. Interdiscip Toxicol 2016;9(2):66-77
https://doi.org/10.1515/intox-2016-0008
.
17. Ellman GL, Courtney KD, Andres V Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7(2):88-95
https://doi.org/10.1016/0006-2952(61)90145-9
.
18. Mousavi SR, Jafari M, Rezaei S, Agha-alinejad H, Sobhani V. Evaluation of the effects of different intensities of forced running wheel exercise on oxidative stress biomarkers in muscle, liver and serum of untrained rats. Lab Animal 2020;49(4):119-125
https://doi.org/10.1038/s41684-020-0503-7
.
19. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72(1–2):248-254
https://doi.org/10.1006/abio.1976.9999
.
20. Van Kampen EJ, Zijlstra WG. Determination of hemoglobin and its derivatives. Adv Clin Chem 1966;8: 141-187
https://doi.org/10.1016/S0065-2423(08)60414-X
.
21. Tahmasebi K, Jafari M, Izadi F, Asgari A, Bahadoran H, Heydari J, et al. Evaluation of prophylactic and therapeutic roles of N-acetylcysteine on biochemical and oxidative changes induced by acute poisoning of diazinon in various rat tissues. Curr Chem Biol 2020;14(2):100-116
https://doi.org/10.2174/2212796814999200818094328
.
22. Eshrati R, Jafari M, Gudarzi S, Nazari A, Samizadeh E, Ghafourian Hesami M. Comparison of ameliorative effects of Taraxacum syriacum and N-acetylcysteine against acetaminophen-induced oxidative stress in rat liver and kidney. J Biochem 2020;169(3):337-350
https://doi.org/10.1093/jb/mvaa107
.
23. Jafari M, Salehi M, Asgari A, Ahmadi S, Abbasnezhad M, Hajihoosani R, et al. Effects of paraoxon on serum biochemical parameters and oxidative stress induction in various tissues of Wistar and Norway rats. Environ Toxicol Pharmacol 2012;34(3):876-887
https://doi.org/10.1016/j.etap.2012.08.011
.
24. Al-Attar AM, Al-Taisan WAA. Preventive effects of black seed (Nigella sativa) extract on Sprague Dawley rats exposed to diazinon. Australian J Appl Sci 2010;4(5):957-968.
25. Ambali SF, Akanbi DO, Shittu M, Giwa A, Oladipo OO, Ayo JO. Chlorpyrifos-induced clinical, haematological and biochemical changes in Swiss albino mice: mitigating effect by co-administration of vitamins C and E. Life Sci J 2010;7(3):37-44.
26. Damodar D, D’Souza UJ, Bhat S. Protective role of Vitamin E: on diazinon-induced hepatotoxicity by biochemical and histological alterations in Wistar rats. Natl J Physiol Pharm Pharmacol 2015;5(5):398-398
https://doi.org/10.5455/njppp.2015.5.2306201569
.
27. Altuntas I, Delibas N. The effects of fenthion on lipid peroxidation and some liver enzymes: the possible protective role of vitamins E and C. Turk J Med Sci 2002;32(4):293-297
https://doi.org/10.1007/s00204-002-0359-1
.
28. Sargazi Z, Nikravesh MR, Jalali M, Sadeghnia HR, Anbarkeh FR. The protective effect of vitamin E on rats’ ovarian follicles following an administration of diazinon: An experimental study. Int J Reprod BioMed 2019;17(2):79-88
https://doi.org/10.18502/ijrm.v17i2.3985
.
29. Jarvik GP, Tsai NT, McKinstry LA, Wani R, Brophy VH, Richter RJ, et al. Vitamin C and E intake is associated with increased paraoxonase activity. Arterioscler Thromb Vasc Biol 2002;22(8):1329-1333
https://doi.org/10.1161/01.ATV.0000027101.40323.3A
.
30. Ambali SF, Akanbi DO, Oladipo OO, Yaqub LS, Kawu MU. Subchronic chlorpyrifos-induced clinical, hematological and biochemical changes in Swiss albino mice: Protective effect of vitamin E. Int J Biol Med Res 2011;2(2):497-503.
31. Shadnia S, Dasgar M, Taghikhani S, Mohammadirad A, Khorasani R, Abdollahi M. Protective effects of α-tocopherol and N-acetyl-cysteine on diazinon-induced oxidative stress and acetylcholinesterase inhibition in rats. Toxicol Mech Methods 2007;17(2):109-115
https://doi.org/10.1080/15376510600860318
.
32. Razavi M, Hosseinzadeh H, Abnous K, Motamedshariaty VS, Imenshahidi M. Crocin restores hypotensive effect of subchronic administration of diazinon in rats. Iran J Basic Med Sci 2013;16(1):64.
33. Kaushik R, Dige M, Dass G, Ramachandran N, Rout PK. Superoxide dismutase activity in response to heat stress in Jamunapari goats. IJBB 2018;55(1):39-43.
34. Salehi M, Jafari M, Asgari A, Salimian M, Abbasnezhad M, Haji HR, et al. Strain-related differences on response of liver and kidney antioxidant defense system in two rat strains following diazinon exposure. Zahedan J Res Med Sci 2016;18(2):e5988
https://doi.org/10.17795/zjrms-5988
.
35. Abd Elmonem HA. Assessment the effect of pomegranate molasses against diazinon toxicity in male rats. J Environ Sci Toxicol Food Technol 2014;8(2):135-141.
36. Nurulain SM, Szegi P, Tekes K, Naqvi SN. Antioxidants in organophosphorus compounds poisoning. Arh Hig Rada Toksikol 2013;64(1):169-176
https://doi.org/10.2478/10004-1254-64-2013-2294
.
37. Ibrahim ATA. Protective role of lycopene and vitamin E against diazinon-induced biochemical changes in Oreochromis niloticus. Afr J Environ Sci Tech 2015;9(6):557-565
https://doi.org/10.5897/AJEST2014.1853
.
38. Shrivastava A, Aggarwal LM, Mishra SP, Khanna HD, Shahi UP, Pradhan S. Free radicals and antioxidants in normal versus cancerous cells-An overview. IJBB 2019;56(1):7-19.
39. Salehi M, Jafari M, Asgari A. Response of liver antioxidant defense system to vitamins E and C against diazinon toxicity in rat. J Sabzevar Univ Med Sci 1970;21(6):1081-1089.
40. Salehi M, Jafari M, Asgari A, Tahmasebi K. Study of protective role of vitamins E and C in reduction of diazinon-oxidative stress in rat kidney. Journal of Ilam University of Medical Sciences 2015;23(3):44-53.
41. Tahmasebi K, Jafari M, Salehi M. Modulation of diazinon-induced oxidative stress by vitamins E and C in rat brain. J Guilan Univ Med Sci 2017;26: 66-73.
42. Javad H, Mahvash J, Saeed K. Comparison of antioxidant properties of N-acetylcysteine and vitamins E and C on diazinon-induced oxidative stress in rat spleen. J Birjand Univ Med Sci 2016;23: 101-109.
43. Abedini MS, Jafari M, Mirzadeh SM, Salem F. The effect of N-acetyl cysteine and vitamins E and C on diazinon-induced oxidative stress in rat erythrocytes. Daneshvar Medicine 2016;23(122):53-63.
44. Boroushaki MT, Arshadi D, Jalili-Rasti H, Asadpour E, Hosseini A. Protective effect of pomegranate seed oil against acute toxicity of diazinon in rat kidney. Iran J Pharm Res 2013;12(4):821.
45. Hariri AT, Moallem SA, Mahmoudi M, Memar B, Hosseinzadeh H. Sub-acute effects of diazinon on biochemical indices and specific biomarkers in rats: protective effects of crocin and safranal. Food Chem Toxicol 2010;48(10):2803-2808
https://doi.org/10.1016/j.fct.2010.07.010
.
Figure 1Effects of diazinon (DZN) with and without vitamin E on plasma biochemical parameters in male Wistar rats 24 h post exposure. Values are expressed as mean±SD (n=6) *p<0.05 and **p<0.01 vs control. AST: Aspartate transaminase; ALT: Alanine transaminase; LDH: Lactate dehydrogenase and ALP: Alkaline phosphatase. 
Figure 2Effects of diazinon (DZN) with and without vitamin E on urea, uric acid and creatinine levels and creatin phosphate kinase (CK) activity in male Wistar rats 24 h post exposure. Values are expressed as mean±SD (n=6) *p<0.05 and ** p<0.01 vs control. 
Figure 3Effects of diazinon (DZN) with and without vitamin E on glutathione (GSH) level in various tissues of Wistar rats 24 h post exposure. Values are expressed as mean±SD (n=6). *p<0.05, ** p<0.01 and *** p<0.001 vs control and # p<0.05 and ## p<0.01 vs DZN. 
Figure 4Effects of diazinon (DZN) with and without vitamin E on malondialdehyde (MDA) level in various tissues of Wistar rats 24 h post exposure. Values are expressed as mean±SD (n=6). *p<0.05 and ** p<0.01 vs control. 
Figure 5Pearson correlation analysis of acetylcholine-esterase (AChE) activity with oxidative stress biomarkers in the brain of Wistar rats 24 h post exposure. SOD: superoxide dismutase; CAT: catalase; GSH: glutathione; MDA: malondialdehyde. 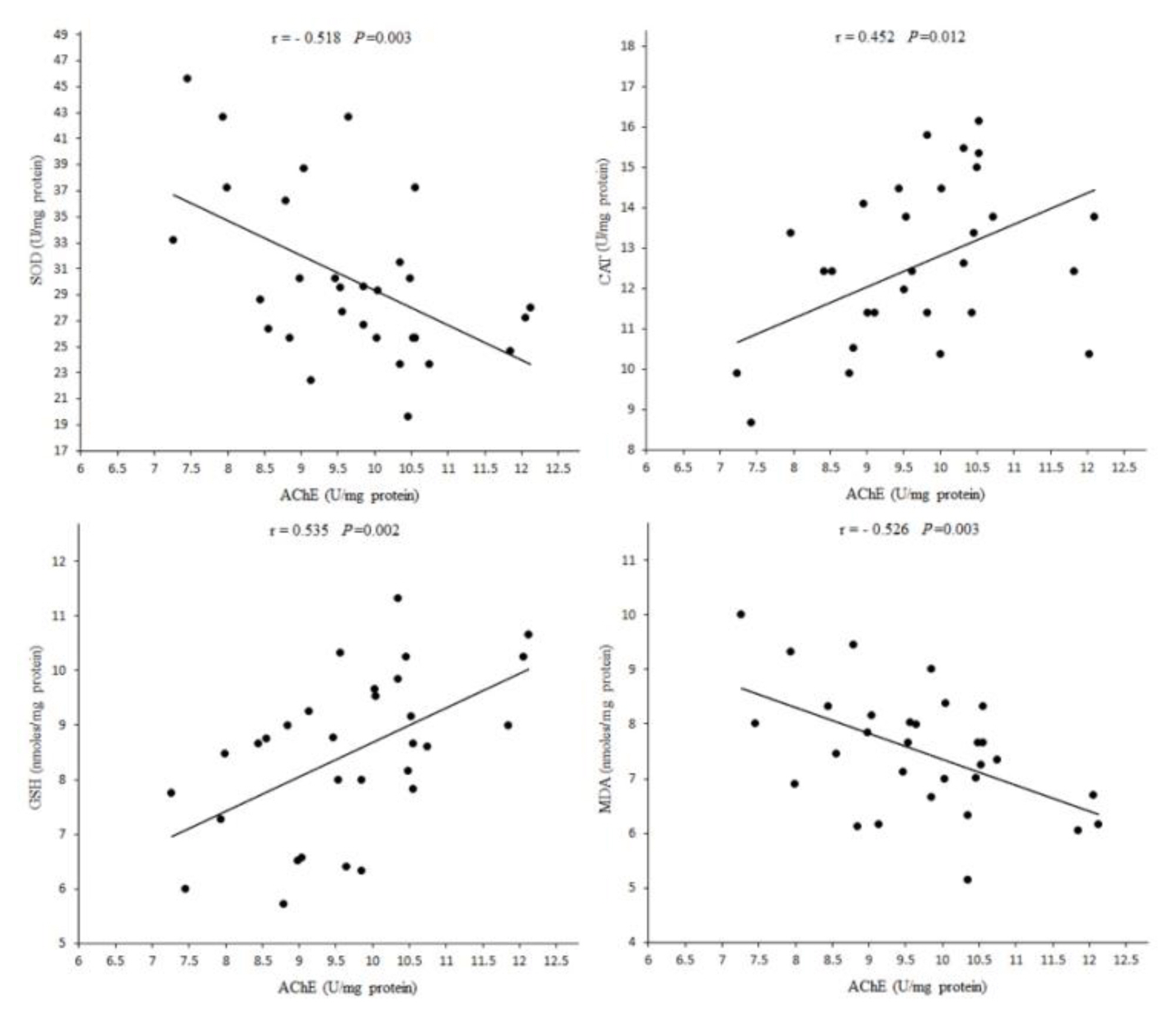
Figure 6
In vivo acute administration of DZN induces the increased lipid peroxidation, alteration in antioxidant enzymes activities and GSH depletion in the tissues, which confirm the involvement of oxidative stress in DZN toxicity. Vitamin E as an antioxidant has beneficial effects against oxidative stress and cholinergic hyperactivity induced by DZN through free radical scavenging. SOD: superoxide dismutase; CAT: catalase and GST: glutathione S-transferase; GSH: glutathione; MDA: malondialdehyde and ChE: Cholinesterase. 
Table 1Effects of diazinon (DZN) with and without vitamin E on AChE (in erythrocytes, brain and spleen), BChE (in liver, kidney and heart) and antioxidant enzyme activities in various tissues of Wistar rats 24 h post exposure.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||