INTRODUCTION
Since 2010, there have been major foot-and-mouth disease cases in Korea. In 2010 and 2011, a total of 3.4 million livestock were buried [1]. Livestock carcasses must be disposed of safely to prevent secondary contamination (odors, pathogens and excess nutrients) [2]. So we need a way to quickly decay livestock carcasses.
Decomposition starts at the moment of death. These processes will release gases that are the chief source of putrid odor of decaying animal tissue [3]. Decomposition of vertebrate animals is carried out in five stages: fresh, bloat, active decay, advanced decay, and dry/remains [4, 5, 6]. First, when the heart attack stops, the fresh phase begins immediately. [7] Within 3 to 6 hours of death, muscular tissues become stern and incapable of relaxing which is known as rigor mortis [8]. From the moment of death, the body loses heat to the surrounding environment. Thus, the overall cooling called algor mortis begins [8, 9]. The process of microbial diffusion in the body is called corruption, leading to the second stage of decomposition known as bloat [7]. The boat stage provides the first clear visual indication of microbial diffusion [10]. In this stage, anaerobic metabolism occurs, leading to accumulation of gases [11]. As the gas pressure in the body increases, the fluid is released from natural holes such as nose, mouth, and anus. It also spreads to the surrounding environment [10, 11]. The build-up of pressure combined with the loss of integrity of the skin may also cause body to rupture [12]. Ruptures in the skin allow oxygen to re-enter the body and supply more surface area for the development of fly larvae and the activity of aerobic microorganisms [12]. The purging of gases and fluids result in powerful and distinctive odors associated with decay [5]. Next, active decay is the most significant feature of mass loss [12]. The build-up of purged fluids around the body produce a cadaver decomposition island (CDI) [7]. At this time, liquefaction and collapse of the tissue appear and the strong smell persists [7, 12]. Decomposition is mostly inhibited during advanced decay due to loss of favorably available cadaveric material [1, 12]. When the carcass is located on soil, the area surrounding it will show evidence of vegetation death [12]. During the last stage, dry/remains stage, dry skin, cartilage, and bones will remain that will dry and become bleached if they are exposed to elements [5, 12]. If all soft tissues are eliminated from the cadaver, it is considered absolutely skeletonized. However, if only sections of bones are exposed, it is referred to as partially skeletonized [13].
Corynebacterium glutamicum is a small, non-pathogenic, non-moving Gram-positive soil bacterium [3]. It is one of important fermentative bacteria widely known for its role in the production of monosodium glutamate (MSG) [14]. C. glutamicum produces several compounds and enzymes with biotechnology applications [15]. Based on the metabolic ability of C. glutamicum, this microorganism was used for livestock carcass disposal [16]. The effects of microorganisms on biomass and enzyme activity have shown that the C. glutamicum, soil microorganism, plays an significant function in the degradation of livestock carcasses [17].
If the livestock is decayed, environmental pollution will occur. Lysosomes possess antimicrobial activity as cell organelles [18]. The use of these lysosomal characteristics can help resolve contamination.
In this study, C. glutamicum as a soil microorganism strain was used to accelerate the decay of livestock carcasses. Furthermore, lysosomes were used to control soil contamination caused by decomposition of livestock.
MATERIALS AND METHODS
Cell culture and soil preparation
C. glutamicum ATCC 13032 (America Type Culture Collection, Manassas, VA, USA) were grown in Luria-Bertani (LB) medium (5 g L-1 yeast extract, 10 g L-1 sodium chloride, and 10 g L-1 trypton) or LB plate (LB medium added with 7.5 g L-1 agar). After growing at 30˚C with shaking (180 rpm) for 36 h in LB medium, approximately 7.8 log10 cfu mL-1 was reached. Cells were then harvested by centrifuging at 3300 rpm for 20 min. Soils were sterilized by autoclave at 121˚C and 1.5 atm for 3 hr.
Experimental condition for laboratory scale of carcass burial
Cultured cells of C. glutamicum were mixed with 10 mL of sterilized water. The mixture of water and C. glutamicum was then mixed with decomposing animal carcasses and soil for burial. We prepared a glass bottle of soil in order to evaluate the decay of animal carcasses in laboratory scale. First, we sterilized soil and glass bottle by autoclave (121˚C, 1.5 atm, 3 hr) for experiment. We placed 50 g of soil in a glass bottle. Feet or wing sample of frozen chickens were purchased from commercial source. They were then added to the 50 g of soil. Chicken legs or wings sample was then treated by C. glutamicum in the glass bottle connected by straw. The shape of the bottle is shown in Figure S1.
Optimization of treatment conditions
We have tried to confirm the optimal conditions for treating C. glutamicum. The cultured C. glutamicum was subjected to the following procedure to treat the conditions for treating the livestock carcass. Treatment time was treated every 10 days and observed from 0 to 50 days. In the presence and absence of air, one container was sealed and one container wasn’t sealed to compare two reactors. To create anaerobic condition, all parts of the reactor were sealed. After covering the top with parafilm without covering the lid, aerobic condition was created by passing air through a hole. Decomposition was monitored through photographs and color change of carcasses (Figure 3). In soil condition, one soil was not put into the container and one soil was put into the container, and the two reactors were compared. The conditions for the reaction of the livestock carcass with C. glutamicum are as follows. The temperature of underground is different depending on the season. When the outside air temperature is -1˚C in the winter, underground temperature is 2-3˚C. In the case of autumn and spring, the outside air temperature is 18˚C and the underground temperature is 17-18˚C. In the summer, when the outside air temperature is 28˚C, the underground temperature is 26-27˚C. Thus, this experiment was performed by selecting 24˚C and 4˚C at similar temperature of summer and winter for soil temperature. The conditions for determining the water content for C. glutamicum for treatment in the reactor were based on the volume of the culture. A piece of whole chickens was then treated by C. glutamicum in soil. This experiment was also monitored every 10 days and observed for a total of 60 days.
Lysosome isolation
Lysosomes were extracted from egg whites. These egg whites were homogenized until the viscosity of egg white was almost zero. The homogenate was centrifuged at 500×g for 5 minutes. The supernatant was then centrifuged again at 20 000×g for 30 minutes [19]. The remaining pellet was collected as lysosomal fraction.
Toxic microbial activity test
The reactors treated with or without C. glutamicum were allowed to rot for 30 days. Lysosomes and C. glutamicum were used to treated carcasses buried in soil after 30 days and allowed to react for 24 hours. After 200 μL of each soil was sampled, sterilized water was mixed with the same volume. After filtering, it was spread on the LB plate.
Data analysis
After treatment with C. glutamicum, to check the degree of decomposition, the sample was photographed every five days. The carcass in the soil was collected on the last day to confirm the extent of degradation through comparing weight before and after decomposition treated by C. glutamicum. The perimeter was measured for the widest part of the carcass without bone.
RESULTS
Optimized time for livestock burial to decompose carcass using C. glutamicum
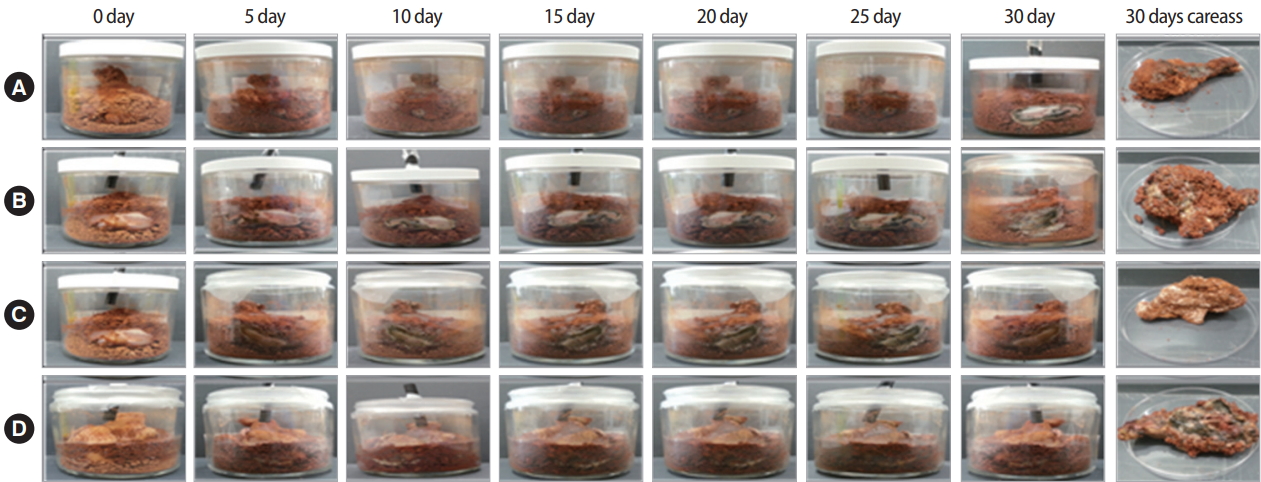
To compare the weight of carcass treated with or without C. glutamicum in reactors, carcasses were taken out the reactors a total of 5 times every 10 days. Results showed that decomposition of carcasses without treatment with C. glutamicum in reactors was delayed for 30 days (Figure 1). In other words, decomposition of the carcass without treatment with C. glutamicum also progressed. However, its speed was slow and the decay process was not complete. For carcass in the reactor treated by C. glutamicum, decomposition was accelerated immediately to bloat stage within 10 days. After decomposition stage was finished, skeletal stage continued from 40 days (Figure 2). Results showed that the weight of carcass was reduced to approximately 20% of its original weight after complete decomposition (Table S1) (A). Circumference changed in carcass and bloated stage gradually occurred for carcasses in all reactors (Figure 1). However, bloated stage happened rapidly until 30 days, entering skeleton stage from 40 days for carcasses treated by C. glutamicum (Table 1). Thus, treatment by C. glutamicum could accelerate decay rate compared to the natural decay process.
Decomposition efficiency by C. glutamicum under various conditions
- Decomposition efficiency of C. glutamicum under anaerobic or aerobic condition
C. glutamicum was cultured under aerobic conditions, although it was usually cultured under anaerobic condition [20]. The purpose of this experiment was to determine the effect of oxygen on the growth of C. glutamicum. To create anaerobic condition, all parts of the reactor were sealed. After covering the top with parafilm without covering the lid, aerobic condition was created by passing air through a hole. Decomposition was monitored through photographs and color change of carcasses (Figure 3). Results showed the weight was changed from 61.23 g to 35.69 g under aerobic condition. Under anaerobic condition, the weight was changed from 61.23 g to 35.69 g (Table S1) (A). Circumference of the carcass treated by C. glutamicum was inflated (Table S1) (B). Under both anaerobic and aerobic conditions, the weight and circumference were increased. Thus, under both anaerobic and aerobic conditions, it was possible to know whether decomposition occurred.
- Decomposition efficiency of C. glutamicum in the presence or absence of soil
We determined the decomposition efficiency of carcasses treated by C. glutamicum in the presence or absence of soil. To confirm the degree of decomposition of carcass according to the presence or absence of soil, after putting chicken sample without soil in the reactor, it was treated C. glutamicum every 10 days. By observing the reactor from the outside, the presence or absence of soil showed no difference (Figure 4). After experiments were carried out for one month, weights of carcasses were determined. The weight of carcass showed no significant difference from 37.83 g initially to 35.62 g after one month (Table S2. (A)). For carcasses without soil, weight of the carcass did not change significantly either after one month (Table S2. (B)). As shown in Figure 4 and Table S2, carcass showed no decomposition. Thus, soil matrix is essential to the decomposition of carcasses by C. glutamicum in this experiment.
- Decomposition efficiency of C. glutamicum in response to temperature
C. glutamicum was cultured under optimal conditions (20 hours, 30˚C, 180 rpm). However, when carcasses are treated in burial, the temperature in the soil is not easy to be maintained at 30˚C. Soil temperature of underground (10-20 m) is not able to change. Since the height of buried land is 3-4 m, the temperature of the soil at actural burial site depends on the outside temperature. The temperature of underground is different depending on the season. The reactor was observed for one month at selected temperature. There was no change in the reactor at 4˚C. However, the color of the soil was changed by decomposition of carcass at 24˚C (Figure S2). In addition, weight of chicken wing was also reduced by almost half (from 24.5 g to 11.3 g) after one month. At 4˚C, the weight was decreased from 33.72 g to 23.1 g after one month. Thus, decomposition was significantly faster at 24˚C (Table S3) (A). Circumference of carcass was also expanded at 24˚C (Table S3) (B).
- Decomposition efficiency of C. glutamicum in accordance with water content
Leachate of outflow in burial site can bring secondary environmental impact such as pollution of groundwater and rivers [21]. We confirmed that carcass was decomposed faster at 24˚C from the above results. Loss of weight did not show big difference after adding 5 ml of distilled water (Figure S3). The reactor showed similar loss rate of weight in case of adding 1 ml or 20 ml of distilled water (Table S4) (A). When water content was 0.01%, circumference was reduced the most (Table S4) (B). We got the best result of decomposition efficiency when 10 ml of distilled water was added to the reactor. Based on these results, such an amount of distilled water was applied to the site and the culture fluid was replaced with a 1/10 volume.
- Decomposition efficiency of sized-up animal treated by C. glutamicum
We would like to know the decomposition efficiency of the whole body treated by C. glutamicum for field applications. By observing the reactor from the outside, we found that the decomposition of whole chicken began between 5 days and 10 days. The amount of distilled water was replaced with a 1/10 volume of the culture medium. We could not distinguish the form of skin surface or muscle tissue in the whole chicken with soil after 60 days (Figure S4).
Post-processing method of livestock burial
After carcass burial, many microorganisms including C. glutamicum will be bred. Toxic microorganisms by decomposition of carcasses can adversely affect the environment. Thus, we determined whether we could get rid of toxic microorganisms by using lysosomes extracted from egg white.
Figure 5. indicates the antibacterial effect of lysosome with or without C. glutamicum for 30 days in soil. Since the weight was increased in the reactor treated by C. glutamicum as described earlier, it was possible to confirm that decomposition had progressed (Table S5). We found that the use of C. glutamicum could rapidly decay carcasses. In addition, lysosome could remove toxic microorganisms adversely affecting the environment.
DISCUSSION
Because corpse processing creates a variety of biological and environmental problems, identifying and using safe and responsible methods is an important factor in maintaining the integrity of the livestock industry and maintaining public health and consumer confidence [22]. C. glutamicum produces several compounds and enzymes with biotechnology applications [15]. Based on the metabolic ability of C. glutamicum, this microorganism was used for livestock carcass disposal [16].
Decomposition starts at the moment of death. These processes release gases that are the chief source of putrid odor of decaying animal tissue [3]. Decomposition of animals has five stages: fresh, bloat, active and advanced decay, and dry/remains [5,6]. We have investigated the corruption effect of C. glutamicum on livestock carcasses through experiments.
In this study, we first evaluated the time dependent stages of decomposition. We confirmed the state of decomposition by taking the carcass out of the reactor. Decomposition of the stage was finished within 30 days, with skeletal stage staring from 40 days. After decomposition, weight was also reduced to about 20% of its original weight. Circumference changed after decomposition. The bloated stage gradually occurred in reactors without treatment with C. glutamicum. However, the bloated stage happened rapidly within 30 days if carcass was treated with C. glutamicum, entering skeleton based from 40 days in reactors. Thus, treatment with C. glutamicum could accelerate the decaying rate of the natural decomposition process.
We examined the effect of temperature, water content, the presence of air, and the presence of soil on the decomposition efficiency by C. glutamicum. Under both anaerobic and aerobic conditions, we found that weight and circumference of carcasses were increased after treatment with C. glutamicum. Thus, decomposition by C. glutamicum occurred well under both anaerobic and aerobic conditions. Regarding the effect of the presence or absence of soil on the decomposition efficiency, the carcass without soil did not change in weight. Thus, the decomposition effect by C. glutamicum needed soil matrix. Experiments were then performed by selecting 24˚C and 4˚C to represent soil status in the summer and winter, respectively. To demonstrate the efficacy of C. glutamicum, temperature and moisture content conditions had to be tailored to some extent. The volume of distilled water to be applied to the site was determined and the culture fluid was replaced with a 1/10 volume.
After carcass burial, a large number of microorganisms, including C. glutamicum, might breed at burial site [23]. Some toxic microorganisms might have adversely affect the environment. Thus, we tried to remove toxic microorganisms by using lysosomes based on a previous study [19]. After using C. glutamicum to rapidly decay carcasses, lysosomes could be used to remove toxic microorganisms adversely affecting the environment.
Therefore, using C. glutamicum, a soil microorganism, can accelerate the decay process of carcass. Furthermore, lysosomes can be used to reduce soil pollution caused by livestock decomposition.