AbstractZinc pyrithione (ZP) is commonly used to prevent dandruff and seborrheic dermatitis. Many consumers are exposed daily to high doses of ZP, causing serious concerns about its toxicity. The reproductive and developmental toxicities were previously reported in pregnant rats. However, the estrogenic activity of ZP at varying degrees of exposure has been rarely studied. Thus, we performed an uterotrophic assay, E-screen assay, and gene expression profiling to assess the estrogenic activity of ZP. For the uterotrophic assay, ZP (2, 10, or 50 mg/kg/d) was subcutaneously administered to ovariectomized rats every day for three days. Uteri were extracted 24 hours after the last dose. Then, wet and blotted uterine weights were measured. For the E-screen essay, MCF-7 cells (a breast cancer cell line) were exposed to 10-9 to 10-6 M of ZP, and cell proliferation was then measured. For the gene expression analysis, changes of gene expression levels in uterine samples taken for the uterotrophic assay were analyzed. In the uterotrophic assay, the concentration of ZP had no significant effect on uterine weight. In the E-screen assay, ZP at any concentration showed no significant increase in MCF-7 cell proliferation, compared to the control group. However, 10-6 M of ZP significantly reduced cell viability. The changes in gene expression slightly differed between the ZP and control groups. The in vivo and in vitro assays, together with gene expression analysis, demonstrated that ZP showed no significant estrogenic activity.
INTRODUCTIONZinc, a divalent cation, is an essential micronutrient for humans. It effectively prevents ultraviolet (UV) damage and reduces the development of malignant tumors. Pyrithione purportedly acts as an ionophore to improve zinc absorption [1,2]. Zinc pyrithione (ZP) inhibits the activity of Malassezia spp., a Eumycota [1,3,4]. It is used alongside calamine and zinc oxide as a photoprotective agent and as an additive in shampoos to treat seborrheic dermatitis and dandruff. Many studies have reported improvement of those conditions after the use of ZP [5- 8]. In the early 1960s, the US Food and Drug Administration (FDA) approved ZP, acknowledging the safety and effectiveness of ZP against dandruff.
While ZP possesses antifungal as well as other positive effects, a number of studies have consistently attributed the development of allergic contact dermatitis and skin side effects to the use of products containing ZP [9]. The Health and Safety Executive of England classifies ZP as a moderate eye irritant, and the MAK Collection for Occupational Health and Safety in Germany considers ZP to be highly corrosive to the eyes [10,11]. In a study on the reproductive and developmental toxicities of ZP, the rate of fused or broken ribs in pregnant rats increased after intravenous administration of ZP [3,12]. Another study reported that pregnant rats experienced reduction in body and uterine weights and paralysis of the hind legs after topical application of ZP [3]. However, in vivo and in vitro toxicological studies regarding the estrogenic activity of ZP are still lacking. Therefore, we selected ZP as the test substance for this study.
To monitor reactions between estrogen receptors and estrogenic chemicals, an uterotrophic assay in rats can be performed by eliminating their endogenous estrogen, exposing them to estrogenic chemicals, and then measuring their uterine weights. Immature and ovariectomized rats are commonly used in this assay [13-15] as recommended by the Organization for Economic Cooperation and Development (OECD), and Environmental Protection Agency (EPA), Endocrine Disruptor Screening and Testing Advisory Committee of US. An E-screen assay measures the increase in proliferation of MCF-7 cells (a breast cancer cell line) to analyze the estrogenic activity of chemicals. Proliferation of MCF-7 cells accounts for an increase in mitotic activity in rodent endometrium [16]. Also, to examine molecular signatures associated with ZP, we performed gene expression profiling of uterine samples from ZP-administrated and estrogen-administrated rats and then identified the genes and also cellular processes affected by ZP and estrogen. The comparison of such genes and cellular processes would show how the effects of ZP and estrogen in uterus were shared at the molecular and cellular process levels [17]. Therefore, all these results can provide critical information regarding whether ZP has the estrogenic activity.
METHODSUterotrophic AssayZP (CAS No. 13463-41-7) was purchased from Tokyo Chemical Industry Co. (TCI; Tokyo, Japan). 17β-Estradiol (E2; CAS No. 50-28-2), used for the positive control, was purchased from Sigma-Aldrich (St. Louis, MO, USA). Corn oil (CAS No. 8001- 30-7), used as a solvent, was purchased from Sigma Life Science (St. Louis, MO, US). Five-week-old Sprague-Dawley (SD) female rats weighing approximately 120 ± 10 g were purchased from Samtako (Osan, Korea).
The rats were allocated to one of five groups: a control group (corn oil), positive control group (10 µg/kg E2), low dose ZP group (2 mg/kg), medium dose ZP group (10 mg/kg), and high dose ZP group (50 mg/kg). Six rats were randomly selected from each of the five groups and placed into cages filled with straw (2 rats per cage). They were raised in a specific pathogen free state in the Laboratory Research Center of Busan University. All rats were freely fed irradiated food (Purina, Seongnam, Korea) and water. The rearing temperature was maintained at 22 ± 1°C with relative humidity at 50 ± 5%, and the light was alternately maintained according to a 12 hours light cycle (8 am to 8 pm).
For twelve days following their arrival in the lab, the rats adapted and acclimatized to the lab environment. Ovariectomy was performed after this 12-day period. Two weeks after recovery from surgery (as recommended by the OECD [13]), we subcutaneously administered ZP diluted in the corn oil solvent to the ovariectomized rats at the same time every day for three days. During the administration period, the dosages (2, 10, or 50 mg/ kg/d) were calculated daily from the weight measurements of the rats. The dose volume was set at 5 mL/kg regardless of the test substance being administered. The control group received only corn oil, while the E2 positive control group received subcutaneous injections of 10 µg/kg E2 diluted in corn oil [18]. The rats were euthanized 24 -hour after the final dose in the order that they received the test substances. The euthanized rats were weighed, and their wet and blotted uterine weights were measured. When necessary, uterine samples were fixed on 10% formalin and liquid nitrogen for microstructure observation and gene expression profiling.
E-screen AssayZP was purchased from TCI, and E2, used for the positive control, was purchased from Sigma-Aldrich. Dimethyl sulfoxide (DMSO), used as a solvent, was purchased from Life Technologies (Carlsbad, CA, USA). MCF-7 cells (Passage No. 176) were purchased from the Korean Cell Line Bank (No. 30022), and were used in the experiment after serial subculture at 37°C, 5% carbon dioxide (CO2).
After sufficient culturing, the MCF-7 cells were counted, and then distributed into a 96-well plate (100 μL per well, 5 × 104 cell/ mL). The plate was lightly shaken to evenly distribute the cells, and then incubated at 37°C, 5% co2 for 24 hours. In the subsequent steps, Dulbecco’s modified Eagle’s medium (DMEM) containing 5% charcoal stripped fetal bovine serum 5% and 1% penicillin streptomycin glutamine was used to eliminate cell proliferation factors. E2 and other test substances were diluted using DMSO as a solvent in the culture medium. Afterward, 100 µL of test substance was added to each group: control group, 0.1% DMEM; E2 positive control group, 1 × 10-9 M E2 [19]; and ZP group, 1 × 10-9 to 1 × 10-6 M ZP. The final concentration of DMSO was maintained at 0.1%. The plate was incubated at 37°C, 5% co2 for 6 days (144 hours). Then, cell proliferation was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay.
Statistical AnalysisSigmaStat (https://systatsoftware.com) was employed to analyze the results from the uterotrophic and E-screen assays. All values were reported as the mean ± standard deviation. One way analysis of variance was used to test the statistical significance of the results. The statistical significance of the test and control groups was tested using a Dunnett’s test, with the level of significance set at p< 0.05 and p< 0.01.
Microarray ExperimentsMicroarray experiments were performed under the following three conditions: 1) corn oil-administrated (control), 2) ZP-administrated, and 3) E2-administrated conditions. In each condition, total RNAs were isolated independently from two biological replicate uterus samples harvested for the uterotrophic assay in different rats using the RNeasy mini kit (Qiagen, Hilden, Germany). The RNA integrity was performed for the isolated RNA using Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA): RNA integrity numbers for all the samples were larger than nine, which were appropriate for gene expression profiling. For each sample, RNA was amplified, reverse-transcribed using reverse transcription polymerase chain reaction (RT-PCR), and then hybridized to the Agilent Rat- 028279, according to Illumina standard protocols. The probe intensities were obtained using SureScan Microarray Scanner (Agilent Technologies). The raw data were deposited in the GEO database (GEO accession ID, GSE89321).
Analysis of Gene Expression ProfilesThe probe intensities were normalized using the quantile normalization procedure. Using the normalized intensities, we performed the following comparisons: 1) ZP-administrated uterus vs. controls; and 2) E2-administrated uterus vs. controls. For each comparison, we used the integrative statistical method previously reported. Briefly, for each gene, we computed a T-value and log2- median-ratio by applying two-tailed t-test and log2-median-ratio test, estimated the empirical distributions estimated for the t-test and log2-median-ratio test by performing random permutations of the samples, and then calculated adjusted p-values for the t-value and log2-median-ratio for the gene. For each gene, the two pvalues from the t-test and log2-median-ratio test were combined to compute the overall p-values using Stouffer’s method. Finally, the differentially expressed genes (DEGs) were selected as the ones having 1) the overall p-value≤0.05; 2) absolute log2-fold changes larger than 0.58 (1.5-fold in the original scale). Cellular processes represented by the DEGs were identified as the gene ontology biological processes with p-value≤0.1 a default cut-off used by the Database for Annotation, Visualization and Integrated Discovery (DAVID) from the Expression Analysis Systematic Explorer method in DAVID software.
RESULTSUterotrophic EffectsWet uterine weights and blotted uterine weights obtained from the uterotrophic assay are listed in Table 1. The wet and blotted uterine weights were significantly higher in the E2 positive control group than in the corn oil control group (6.7 and 2.7 times higher, respectively). Those volume differences were visually observable (Figure 1). Uterine weights remained unchanged after three consecutive days of subcutaneously administration of 2, 10, and 50 mg/kg/d ZP (Figure 2).
E-screen AssayResults of the E-screen assay are shown in Table 2. Proliferation of MCF-7 cell was significantly higher in the E2 positive control group than in the control group (0.1% DMSO) (Figure 3). No significant increase in MCF-7 cell proliferation was observed at 1 × 10-9 to 1 × 10-6 M ZP compared to that of the control group. However, we noted that cell viability was significantly reduced at 1 × 10-6 M ZP.
Gene Expression ProfilingBoth the uterotrophic and E-screen assays revealed that the administration of ZP had no significant activity of estrogen. To confirm whether ZP had no significant shared effect with E2 at the molecular level, we performed gene expression profiling of uterus samples from ZP-administrated and E2-administrated rats and then performed the following two comparisons using the resulting gene expression profiles: 1) ZP-administrated samples vs. controls and 2) E2-administrated samples vs. controls. From the two comparisons, we identified the genes affected by ZP and E2 as 398 (218 up-regulated and 180 down-regulated) and 4738 (2163 up-regulated and 2575 down-regulated) DEGs in ZP-administrated and E2-administrated samples, compared to controls, respectively. To examine overlapping DEGs by considering their up-regulation and down-regulation together, we further categorized the DEGs into eight groups based on their differential expression patterns in the two comparisons. This categorization revealed 91 up-regulated and 104 downregulated genes showing shared up-regulation and down-regulation in the two comparisons. Thus, the two sets of the DEGs showed a significant (p< 0.01) number of shared up-regulated and down-regulated genes (195 of 398 genes affected by ZP, 49.0%), suggesting that a significant portion of the response to ZP was shared with that to E2 at the transcript level.
To understand whether cellular processes affected by ZP and E2 were also shared, we performed the enrichment analysis of gene ontology biological processes (GOBPs) for up-regulated and down-regulated genes by ZP and E2 using DAVID software. Of the GOBPs affected by E2, the processes related to hormone response (regulation of hormone levels and response to steroid hormone stimulus), metabolism (sterol/steroid and hexose metabolic processes), and protein folding were commonly up-regulated by ZP and E2 (Table 3). On the other hand, the processes related to hypoxic response (response to oxygen levels) were commonly down-regulated by ZP and E2. These GOBPs represented by the up-regulated and down-regulated genes could be considered as general stress responses to a chemical, such as ZP. Interestingly, however, in E2-administrated samples, the estrogen responses, more relevant to the estrogen activity, were represented by both up-regulated and downregulated genes by E2. In contrast, of them, only the responses represented by the down-regulated genes were shared between E2 and ZP. Taken together, these data suggest that ZP does not contribute to up-regulation of the estrogen response, but rather down-regulation of the estrogen response.
DISCUSSIONAn uterotrophic assay of ovariectomized rats (an in vivo estrogenic activity screening method recommended by the OECD) showed that subcutaneous administration of ZP at 2, 10, and 50 mg/kg/d did not affect uterine weights. Moreover, an E-screen assay (an in vitro estrogenic activity screening method) of MCF- 7 cells exposed to 1 × 10-9 to 1 × 10-6 M ZP did not show an elevation in estrogenic activity (i.e., MCF-7 cell proliferation did not increase). In fact, MCF-7 cell viability significantly declined at 1 × 10-6 M ZP. When the uterine samples collected for the uterotrophic assay were analyzed, we discovered that changes in gene expression in response to the hormone stimulus pathway were similar between the ZP and E2 groups.
In the uterotrophic and E-screen assays, no estrogenic activity for ZP was found. When combined with gene expression analysis, the findings indicate that changes in gene expression related to increased uterine weight do not necessarily correspond with estrogenic activity. ZP was tested estrogen receptor bioactivity from US EPA using Endocrine Disruptor Screening Program (EDSP). It showed ZP has some estrogenic activity (ER bioactivity, 0.237) [20]. According to the results, ER bioactivity of E2 was 0.935, bisphenol A 0.450, butyl paraben 0.251, 4-octylphenol 0.118, and 4-nonylphenol 0.088, among the substances known to be endocrine disruptors. But EDSP data do not provide a scientific basis, just supporting a conclusion that substances have potential for endocrine disruption. The endocrine disruption of chemicals should be evaluated by concluding comprehensive assessments of various in vitro and in vivo tests. Although we conclude that ZP does not have estrogenic activity, additional research into the neurotoxicity and skin toxicity of ZP is necessary for determining the safety of ZP.
ACKNOWLEDGEMENTSThis research was financially supported by Changwon National University in 2015-2016.
Conflict of interestThe authors have no conflicts of interest associated with the material presented in this paper.
REFERENCES1. Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract 2014;2014: 709152.
2. Bibi Nitzan Y, Cohen AD. Zinc in skin pathology and care. Zinc in skin pathology and care. J Dermatolog Treat 2006;17(4):205-210.
3. Scientific Committee on Consumer Safety. Opinion on zinc pyrithione: COLIPA n° P81;. 2013. [cited 2017 Feb 15]. Available from: file:///C:/Users/user/Downloads/NDAQ13004ENN_002.pdf.
4. Gupte TE, Gaikwad UV, Naik SR. Experimental studies (in vitro) on polyene macrolide antibiotics with special reference to hamycin against Malassezia ovale. Comp Immunol Microbiol Infect Dis 1999;22(2):93-102.
5. Warner RR, Schwartz JR, Boissy Y, Dawson TL Jr. Dandruff has an altered stratum corneum ultrastructure that is improved with zinc pyrithione shampoo. J Am Acad Dermatol 2001;45(6):897-903.
6. Rapaport M. A randomized, controlled clinical trial of four antidandruff shampoos. J Int Med Res 1981;9(2):152-156.
7. Marks R, Pearse AD, Walker AP. The effects of a shampoo containing zinc pyrithione on the control of dandruff. Br J Dermatol 1985;112(4):415-422.
8. Food and Drug Administration, HHS. Dandruff, seborrheic dermatitis, and psoriasis drug products containing coal tar and menthol for over-the-counter human use; amendment to the monograph. Final rule. Fed Regist 2007;72(43):9849-9852.
9. Hsieh CW, Tu ME, Wu YH. Allergic contact dermatitis induced by zinc pyrithione in shampoo: a case report. Dermatol Sin 2010;28(4):163-166.
10. Health and Safety Executive. Evaluation on: zinc pyrithione: use as a booster biocide in antifouling products. Bootle: Advisory Committee on Pesticides; 2003.
11. Commission for the Investigation of Health Hazards of Chemical Compounds. The MAK-collection for occupational health and safety;. 2012. [cited 2017 Feb 15]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/3527600418.mb1346341d0052/pdf (German).
12. Haley S, Plank JB, Wright FL, Keplinger ML. Teratology study with zinc Omadine in albino rats. Clayton: Industrial Bio-Test laboratories; 1971.
13. Organization for Economic Cooperation and Development. Test No. 440: uterotrophic bioassay in rodents: a short-term screening test for oestrogenic properties;. 2007. [cited 2017 Feb 15]. Available from: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg440.pdf.
14. Han SY, Moon HJ, Kim HS, Kim CK, Shin JH, Oh SD, et al. No estrogenic activity of phthalate esters in ovariectomized rat uterotrophic assay. J Appl Pharmacol 2000;8(2):147-152 (Korean).
15. Owens W, Ashby J, Odum J, Onyon L. The OECD program to validate the rat uterotrophic bioassay. Phase 2: dietary phytoestrogen analyses. Environ Health Perspect 2003;111(12):1559-1567.
16. Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. The E-screen assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect 1995;103 Suppl 7: 113-122.
17. Kim S. Microarray gene expression analysis. Korean J Clin Microbiol 2001;4(2):82-86 (Korean).
18. Odum J, Lefevre PA, Tittensor S, Paton D, Routledge EJ, Beresford NA, et al. The rodent uterotrophic assay: critical protocol features, studies with nonyl phenols, and comparison with a yeast estrogenicity assay. Regul Toxicol Pharmacol 1997;25(2):176-188.
19. Kwack SJ, Kwon O, Kim HS, Kim SS, Kim SH, Sohn KH, et al. Comparative evaluation of alkylphenolic compounds on estrogenic activity in vitro and in vivo. J Toxicol Environ Health A 2002;65(5-6):419-431.
20. US Environmental Protection Agency. Endocrine Disruptor Screening Program (EDSP) estrogen receptor bioactivity. [cited 2016 Dec 31]. Available from:https://www.epa.gov/endocrinedisruption/endocrine-disruptor-screening-program-edsp-estrogen-receptor-bioactivity.
Figure 1.Uterine appearances in response to (A) control, (B) 17β -estradiol 10 µg/kg/d, (C) ZP 2 mg/kg/d, (D) ZP 10 mg/kg/d, and (E) ZP 50 mg/ kg/d. ZP, zinc pyrithione. 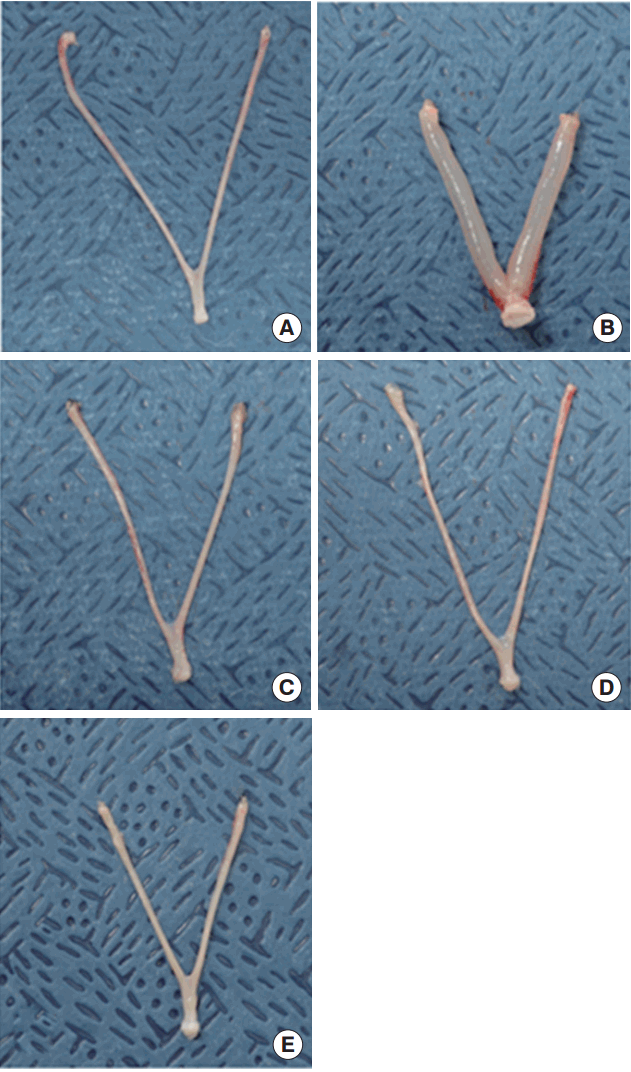
Figure 2.The effect of ZP on the uterine weight of Sprague Dawley rats in the uterotrophic assay. The control group received only corn oil. Data represent wet & blotted uterine weight of six animals per group. OVx, control group; E2, 17β-estradiol; ZP, zinc pyrithione. *p<0.05, **p<0.01. 
Figure 3. Effects of ZP on the proliferation of MCF-7 human breast cancer cells. Cells were exposed for 6 days to ZP (1×10-9 to 1×10-6 M) or E2 (1×10-9 M) in Dulbecco’s modified Eagle’s medium supplemented with 5% charcoal stripped fetal bovine serum. Cell proliferation was determined by 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide assay. E2, 17β-estradiol; ZP, zinc pyrithione.**p<0.01. 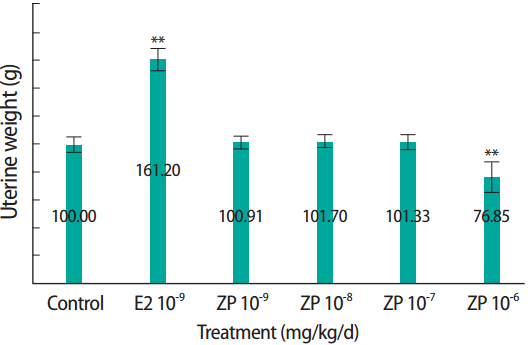
Table 1.The effect of ZP on the uterine weight of Sprague-Dawley rats in the uterotrophic assay
Table 2.The effect of ZP on MCF-7 cell proliferation in the E-screen assay
Table 3.GOBPs1 represented by up- and down-regulated genes by ZP or E2 DEGs |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||