Introduction
Breast cancer is the most common disease for women in Korea [1]. Because estrogens are the main determinants in breast cancer, aromatase, the key enzyme of estrogen synthesis, has been considered one of the most important enzymes among the steroidogenesis enzymes [2,3]. Therefore, aromatase inhibitors (AIs) such as anastrozole, letrozole, and exemestane are used to treat breast cancer by depleting estrogen [3,4]. However, although AIs have been proven as a standard endocrine therapy medicines based on large clinical trials, they have limitations because of their side effects, which include bone fracture, bone loss, and musculoskeletal events [5]. These limitations of synthetic medicines have spurred efforts to search for new compounds that could be effective at tolerable doses without adverse effects [6].
Ginkgo biloba is a tree that has been used in traditional Chinese medicine for about 5000 years and is now commercially cultivated in France and the US [7]. Previous studies reported that Ginkgo biloba extract (EGb) has a biphasic effect on estrogen activity [8,9] and bone density through osteoblastic and anti-osteoclastic effects [10]. EGb and its major constituents (quercetin and kaempferol) were reported to regulate estrogen levels as aromatase inhibitors [11,12]. In addition, Park et al. [13] elucidated that standardized extract of Ginkgo biloba (EGb761) could induce anti-carcinogenic effects on breast cancer as the aromatase inhibitors. However, the effects of EGb761 on entire steroidogenesis pathways (aldosterone, cortisol, testosterone, and 17β-estradiol synthesis) have not been studied yet. Therefore, in this study, the effects of EGb761 on steroidogenesis pathways and aromatase activities were evaluated.
In postmenopausal women, the steroidogenic enzymes are mainly expressed in the adrenal glands, which are the main source of estrogens. The human H295R adrenocortical carcinoma cells have the physiologic features of zonally undifferentiated human fetal adrenal cells and express all the steroidogenic enzymes essential for steroid hormone synthesis in the adult adrenal cortex [14]. As such, the H295R cell line has been used broadly as an in vitro model to predict effects on human adrenal steroidogenesis [15,16]. In this study, the H295R cell model was used to evaluate effects of EGb761 on steroidogenesis pathways. First, a cell viability test was carried out to find the appropriate dose-range of EGb761 without cytotoxicity. Second, levels of four steroid hormones including cortisol, aldosterone, testosterone, and 17β-estradiol, were measured by competitive enzyme- linked immunospecific assay (ELISA). Third, the genes required for steroid hormone production including steroidogenic acute regulatory protein (StAR), cytochrome P450 (CYP11, CYP17, CYP19, and CYP21), and the hydroxysteroid dehydrogenases (3β-HSD2, 17β-HSD1, and 17β-HSD4) were quantified by real-time polymerase chain reaction (PCR). Finally, aromatase activity was measured with a tritiated water release assay and protein levels were measured by western blotting.
Materials and Methods
Cell Culture
H295R human adrenocarcinoma cells (CRL-2128) were purchased from the American Type Culture Collection (Fairfax, VA, USA) and cultured as a monolayer in H295R supplemented media, which is a 1:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F-12 nutrient mixture (DMEM/F12) (Sigma- Aldrich, St. Louis, MO, USA) containing 1% ITS + Premix (BD Biosciences, San Diego, CA, USA), 2.5% Nu-Serum (BD Biosciences), 1.2 g/L Na2CO3 (Sigma-Aldrich), and 1% penicillin- streptomycin (GibcoBRL, Grand Island, NY, USA). Cells were cultured in a humidified environment at 37°C containing 95% air/5% carbon dioxide. Medium was changed three times a week and cell subculture was performed every seven days.
Test Chemicals
The EGb761 were kindly provided and standardized by YuYu Pharma Inc. (Seoul, Korea). The EGb761 contained 24.7% flavones glycosides (including quercetin, kaempferol, and isorhamnetin) and 6.1% terpene lactones (3.2% ginkgolides and 2.9% bilobalide), which represent the major active components of EGb761. The solution was prepared in 50% ethanol/water at 250 mg/mL. Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich. The radioactive solution, [1β-3H] androst- 4-ene-3,17-dione, was purchased from PerkinElmer® (Waltham, MA, USA).
Cell Viability Test
To evaluate cell viability and to exclude cytotoxic concentration ranges of EGb761, the cells were seeded onto 96-well plates at a density of 10000 cells/well with 200 μL of medium. After incubation with EGb761 (from one to 1000 μg/mL) for 24 hours, cell viability was measured by the WST-1 assay (Roche, Mannheim, Germany).
Hormone Measurement (Aldosterone, Cortisol, Testosterone, and 17β-estradiol)
Cells were seeded into 24-well plates at a density of 30×104 cells/mL in 1 mL of medium per well. After 24 hours, the cells were exposed to EGb761 (10 and 100 μg/mL) for 24 hours. In instances where exposure resulted in cell viability of less than 85%, the cells were not used for the evaluation of hormone production [17]. Hormones were measured by competitive ELISA following the manufacturer’s recommendations (Cayman Chemical, Ann Arbor, MC, USA; aldosterone [Catalog no. 1004377], cortisol [Catalog no. 500360], testosterone [Catalog no. 582701], and 17β -estradiol [Catalog no. 582251]). The detection limit of aldosterone, cortisol, testosterone, and 17β-estradiol were 24.02, 14.97, 11.71, and 22.75 pg/mL, respectively.
Quantitative Real-time Polymerase Chain Reaction on Genes Related to Steroidogenesis in H295R Cells
To characterize the regulation of gene expression in cells, we performed real-time PCR using specific sense and anti-sense primers, in order to check the mRNA expression levels. Total RNA was purified from cells exposed to EGb761 (10 and 100 μg/mL) for 24 hours with Trizol® (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized from 2 μg of total RNA using AMV reverse-transcriptase (Promega, San Luis Obispo, CA, USA) in a 20 μL reaction. Quantitative real-time PCR reaction mixtures (15 μL) contained 1 μL (0.1 μM) of forward and reverse primers, 3 μL of cDNA sample, and 7.5 μL of 2x SYBR Green™ PCR Master Mix. The following thermal cycle profile was used: denaturing at 94°C for 10 minutes, followed by 40 cycles of denaturing for 15 seconds at 94°C, annealing with extension at 60°C to 64 °C (Table 1) for one minute, 72°C for 30 seconds, respectively, and a final cycle of 94°C for 15 seconds, 50°C for one minute, and 94°C for 15 seconds. The primer sequences were synthesized by Bioneer (Daejeon, Korea) and are shown in Table 1. Expression levels of mRNA were quantified by use of the threshold cycle (Ct) method. Ct values for each gene of interest were normalized to β-actin.
‘In-cell’ Aromatase Activity Assay - Tritiated Water-Release Assay
Indirect and direct effects on aromatase activity were measured using a tritiated water-release assay as described by Lephart and Simpson [18] with minor modifications. This assay was performed by measuring the amount of [3H]H2O released upon the conversion of [1β-3H]androst-4-ene-3,17-dione to estrone [18,19]. Cells were plated in 24-well plates with growth media and exposed to EGb761 (10 and 100 μg/mL) in the incubation media after cells were 80% confluent. The incubation media were prepared in 0.1% (v/v) ethanol in H295R supplemented media for H295R cells. The experiments were conducted in three different ways: (1) Indirect aromatase activity: cells plated in 24-well plates were exposed to EGb761 (10 and 100 μg/mL) for 24 hours and then the cells were incubated in 0.25 mL of supplemented medium containing 54 nM [1β-3H] androst- 4-ene-3,17-dione (PerkinElmer®) for 2 hours; (2) Direct aromatase activity: untreated cells were exposed to EGb761 (10 and 100 μg/mL) while a 2 hours tritium-release assay was conducted to assess direct interaction with the enzyme. Aromatase enzyme activity was measured as described by Kim et al. [11].
Western Blot Analysis
Protein from the cells exposed to EGb761 (10 and 100 μg/mL) was extracted with radioimmuno-precipitation assay (RIPA; Thermo Scientific, Rockford, IL, USA) buffer containing 1% protease inhibitor cocktail solution (GenDEPOT, Houston, TX, USA). Lysate proteins (50 μg) denatured with 2x sample buffer (Bio-RAD, Hercules, CA, USA) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose blotting membranes. Immunodetection was performed using anti-aromatase (1:1000, abcam35604, Abcam Plc, Cambridge, UK) and anti-actin antibodies (1:4000, 13E5, Cell Signaling Technology, Danvers, MA, USA), respectively. The corresponding anti-rabbit horseradish peroxidase (HRP) conjugated secondary antibody (Abcam Plc) was used. An ECLplus detection kit (Thermo Fisher Scientific Inc., Rockford, IL, USA) provided the chemiluminescence substrate for HRP, and the targeted protein was visualized by autoradiography.
Statistical Methods
SigmaPlot version 12.0 ( Jandel Scientific, San Rafael, CA, USA) and Excel 2013 (Microsoft Co., Redmond, WA, USA) were used to analyze the data. The data from each assay were expressed as mean ± standard deviation. Statistical analysis was performed using the SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) program. Differences between groups were tested by Duncan’s post-hoc test following a one-way analysis of variance (ANOVA). Statistical significance was accepted at p<0.05.
Results
Cell Viability Effects of Standardized Extract of Ginkgo biloba
Since cytotoxic concentrations should be excluded from the experiments, we first evaluated cell viability following exposure to various concentrations of EGb761 with the WST-1 assay. Cell viability was significantly inhibited at 750 and 1000 μg/mL EGb761. The observed viability was greater than 85% in the EGb761 concentration range of 10 to 100 μg/mL (Figure 1), which then was chosen as the concentration range to be used in the experiments.
The Effects of Standardized Extract of Ginkgo biloba on Hormone Concentrations in H295R Cells
As shown in Figure 2, exposure to EGb761 (10 and 100 μg/mL) resulted in a significant decrease in 17β-estradiol (diamond symbol) and testosterone production (square symbol). However, cortisol (triangle symbol) and aldosterone (circle symbol) levels were not significantly changed in H295R cells exposed to 10 and 100 μg/mL of EGb761.
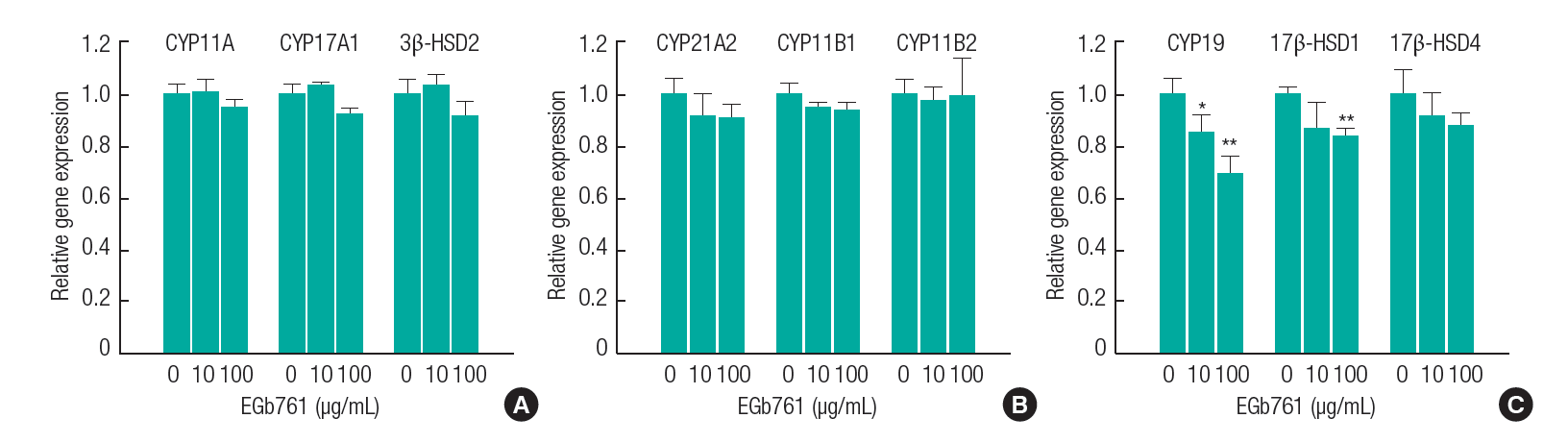
The Effects of Standardized Extract of Ginkgo biloba on Expression of Enzymes Related to Steroidogenesis Pathways
As shown in Figure 3, the mRNA expression of nine genes, including CYP11A, CYP17A1, 3β-SD2, CYP21A2, CYP11B1, CYP11B2, CYP19, 17β-HSD1, and 17β-HSD4 were evaluated in H295R cells exposed to EGb761. The mRNA expression of aldosterone/cortisol-related genes such as CYP11A, CYP17A1, 3β-HSD2, CYP21A2, CYP11B1, CYP11B2, and 17β-HSD4 showed no significant changes in response to EGb761 exposure. However, the mRNA expression of CYP19 and 17β-HSD1, which are related to 17β-estradiol and testosterone synthesis, were significantly decreased by EGb761.
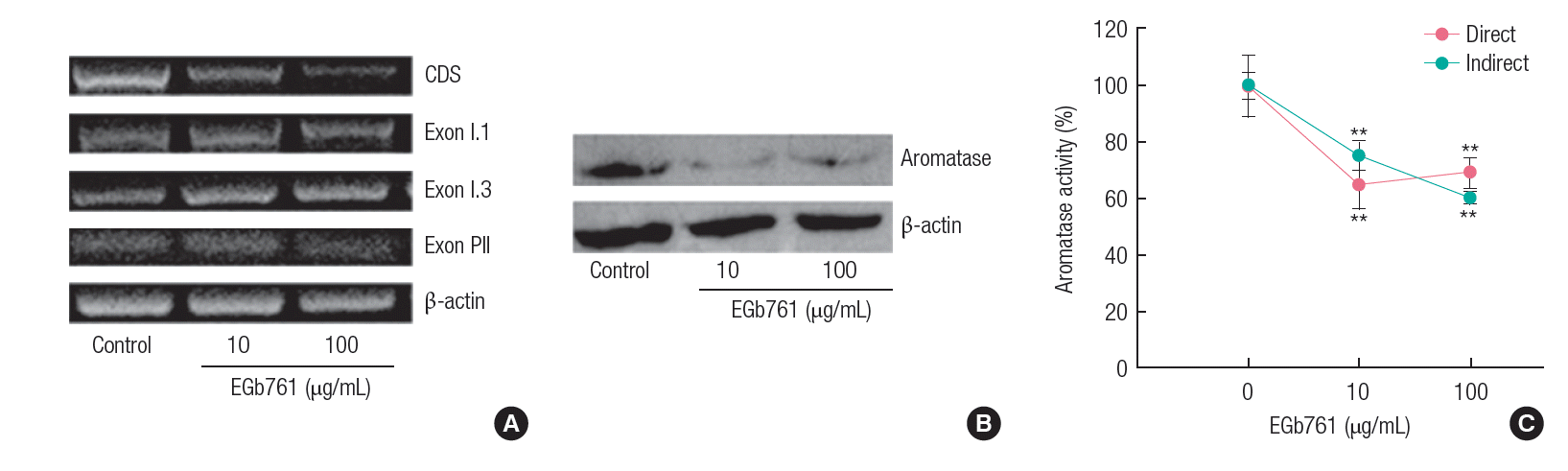
Effects of Standardized Extract of Ginkgo biloba on Aromatase Activity
As shown in Figure 4A, the expression level of the aromatase coding region sequences (CDS) as well as promoter region sequences (Exon PII) were decreased by EGb761. Western blotting confirmed that CYP19 protein synthesis was reduced by EGb761 exposure (Figure 4B). In addition, aromatase activity measured by both direct and indirect methods was significantly inhibited by exposure to EGb761 (10 and 100 μg/mL) when compared with control (Figure 4C).
Discussion
The effects of EGb761 on the synthesis of the four steroid hormones (aldosterone, cortisol, testosterone, and 17β-estradiol) were evaluated using the human H295R adrenocortical cell line as an in vitro model. In the H295R cells exposed to EGb761 at non-cytotoxic concentrations (10 and 100 μg/mL, Figure 1), testosterone and 17β-estradiol were significantly decreased, whereas aldosterone and cortisol were not (Figure 2).
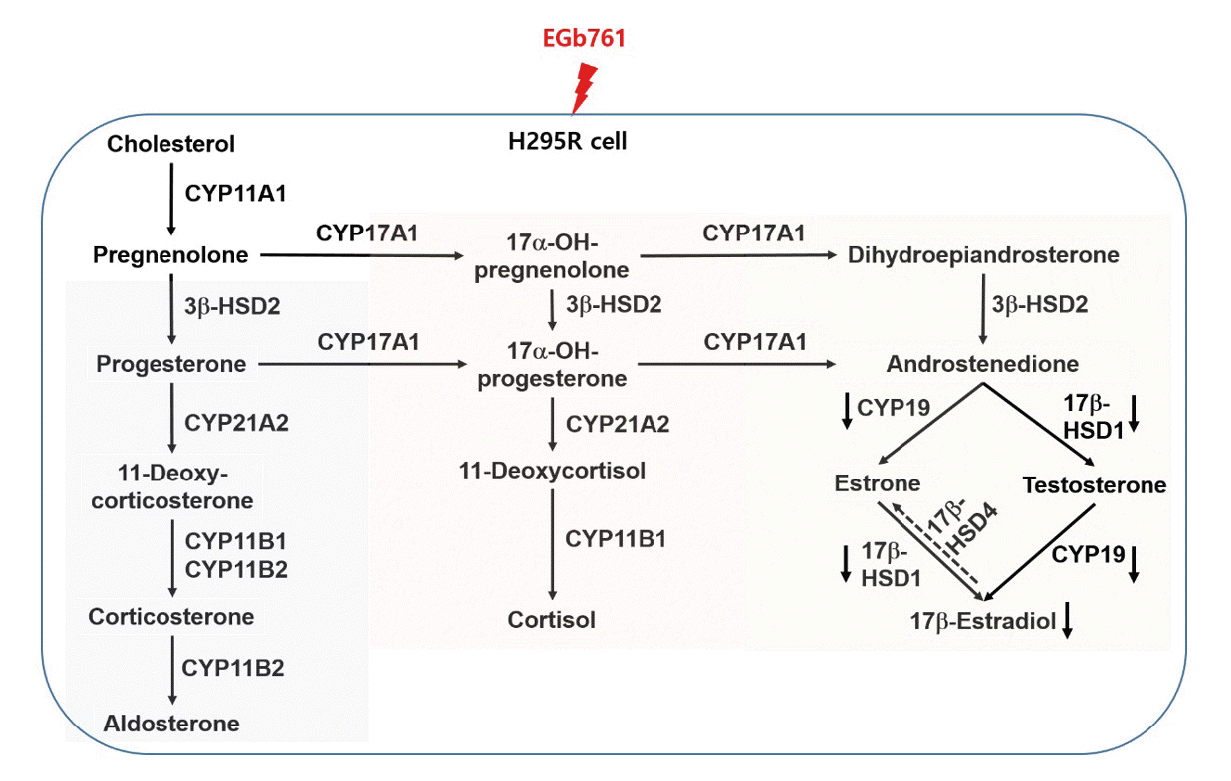
These steroid hormones are synthesized from cholesterol by a series of steroidogenesis pathways, which are dependent on cytochrome P450 enzymes and HSDs [20]. As the first step in the steroidogenesis pathway, cholesterol transported into the inner mitochondrial membrane by StAR [21] is converted into pregnenolone by the cholesterol side-chain cleavage enzyme (CYP- 450scc), which is known as CYP11A1 [16]. In addition, aldosterone and cortisol are synthesized by regulation of the CYP17A1, 3β-HSD2, CYP21A2, and CYP11B1/2 enzymes. In real- time PCR analysis, EGb761 exposure did not affect the expression of these genes (Figure 3), which was consistent with the results showing no change in aldosterone and cortisol levels in H295R cells exposed to EGb761. Although the cortisol level was not significantly changed by EGb761 exposure in this in vitro model system (H295R cell line), several researchers have reported regulation of the cortisol level by EGb761 exposure. Kudolo [22] reported that ingestion of EGb has an effect on the hypothalamic-pituitary-adrenal (HPA) axis leading to reduced basal cortisol levels and reduced cortisol production in response to an acute hyperglycemic challenge. In addition, EGb761 may inhibit cortisol release in response to some stress stimuli [23]. The H295R assay used in this study is not intended to identify substances that affect steroidogenesis due to effects on the hypothalamic- pituitary-gonadal and HPA axes [19], which may produce different outcomes between in vitro and in vivo cortisol levels.
In regard to the steroidogenesis pathway (Figure 5), testosterone and 17β-estradiol levels can be regulated by 17β-HSD1 and CYP19. CYP19, which is related to aromatase activity, is the key enzyme responsible for the final rate-limiting reaction in 17β-estradiol production [24]. 17β-HSD1 is the most well characterized of the 17β-HSDs catalyzing the reaction from estrone to estradiol [25], whereas 17β-HSD4, as an oxidative enzyme, catalyzes the conversion of estradiol to estrone [26]. In this study, EGb761 treatment of H295R cells resulted in a significant reduction of testosterone and 17β-estradiol levels (Figure 2), which was consistent with the results showing a significant decrease in 17β-HSD1 and CYP19 at the concentrations tested (Figure 3). Generally, an increased level of 17β-estradiol could stimulate the proliferation of the tumor tissue via the estrogen receptor, leading to estrogen-dependent breast cancer. In breast cancer patients that have high 17β-HSD1 and CYP19 expression, inhibition of these enzymes may provide therapeutic benefit by depriving the tumor of 17β-estradiol [27,28]. Therefore, we suggest that EGb761 should be evaluated as a therapeutic agent against estrogen-dependent breast cancer, as an inhibitor of 17β-HSD1 and CYP19.
EGb761 showed stronger inhibition of CYP19 gene expression than of 17β-HSD1 (Figure 3). The regulation of the CYP19 gene is controlled by several distinct tissue-specific promoters, which are associated with the placenta (I.1 and 1.2a), skin/normal breast adipose tissue (I.4), endothelial cells (I.7), brain (I.f), bone (I.6), fetal tissues (I.5), adipose tissue (I.4), endometriosis/ ovary (II), and breast tumors (I.7 and I.3/II) [29,30]. These promoters regulate the transcription of the aromatase gene in human tissues [31]. H295R cells showed aromatase activation by promoter 1.3/II-specific aromatase transcripts, but did not detect aromatase activity by promoter 1.4 [32]. In this study, H295R cells contained Exon I.1, I.3 and PII, and EGb761 showed a significant decrease of only PII transcript (Figure 4A), which is the most active promoter of aromatase gene expression in H295R cells [32,33]. In previous studies, EGb761 inhibited expression of Exon 1.3/PII in MCF-7 AROM cells [13] and Exon 1.1 in human placenta JEG-3 cells [11,12]. These results indicated that EGb761 could regulate tissue- specific transcription of the aromatase gene.
The aromatase activities in H295R cells exposed to EGb761 were significantly inhibited by both the direct and indirect pathways (Figure 4C). Direct interactions with the aromatase enzyme, which had the typical inhibitory action, can include competition of the substance with the ligand, or interference with the conversion process of testosterone to 17β-estradiol. On the other hand, indirect inhibition is associated with gene expression or feedback mechanisms [19]. Direct inhibition of aromatase activity by EGb761 was consistent with the results in MCF- 7 AROM cells exposed to EGb761 [13]. In addition, the significant inhibition of CYP19 mRNA and protein levels could support indirect inhibition of aromatase activity by EGb761 exposure. Therefore, we suggest that a significant decrease of 17β-estradiol by EGb761 could be induced by the inhibition of aromatase activity by both the indirect and direct pathways.
Among the chemicals displaying a strong inhibitory effect on hormone secretion, a few have been used clinically in the treatment of adrenal disorders and as steroidogenic inhibitors [34]. Aminoglutethimide, a “generation I” aromatase inhibitor, has been reported to act as a potent inhibitor of CYP450scc. Due to its direct inhibition of CYP450scc and the aromatase enzyme, it was used to treat Cushing’s syndrome (a disease that causes an increase in cortisol) and breast cancer, respectively [35,36]. Exposure to aminoglutethimide resulted in a decrease of testosterone and 17β-estradiol [15]. This mechanism is consistent with the results showing decreased testosterone and 17β-estradiol production by EGb761, which was expected due to the inhibition of 17β-HSD1 and CYP19, respectively. Therefore, these aminoglutethimide results support the assertion that EGb761 also could be an effective natural inhibitor of aromatase as well as steroidogenic enzymes and hormones.
In conclusion, regarding steroidogenesis in H295R cells, EGb761 significantly decreased CYP19 and 17β-HSD1 gene expression, which are related to sex steroid hormones. Specifically, inhibition of the aromatase enzyme via direct and indirect pathways in H295R cells exposed to EGb761 could induce a significant decrease of 17β-estradiol secretion. In addition, EGb761 showed no change on the secretion of other steroid hormones such as aldosterone and cortisol. Down-regulation of CYP19/17β-HSD1 and depletion of 17β-estradiol and testosterone have been known to play an important role in hormone dependent breast cancer. Therefore, we suggest that EGb761 should be evaluated as a therapeutic agent in hormone dependent breast cancer because of its specific sex steroid hormone down-regulation.