AbstractObjectivesThe present subacute study was designed to evaluate the effect of coenzyme Q 10 (CoQ10) in the 28 days aroclor 1254 exposure induced oxidative stress in mice brain.
MethodsBiochemical estimations of brain lipid peroxidation (LPO), reduced glutathione (GSH), and activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and acetyl cholinesterase (AChE), and histopathological investigations of brain tissue were carried out.
ResultsOral exposure of aroclor 1254 (5 mg/kg) led to significant decrease in levels of GSH, and activities of SOD, CAT, GPx, and AChE, and increase in LPO. These aberrations were restored by CoQ10 (10 mg/kg, intraperitoneal injection [IP]). This protection offered was comparable to that of L-deprenyl (1 mg/kg, IP) which served as a reference standard.
ConclusionsAroclor 1254 exposure hampers the activities of various antioxidant enzymes and induces oxidative stress in the brains of Swiss albino mice. Supplementation of CoQ10 abrogates these deleterious effects of aroclor 1254. CoQ10 also apparently enhanced acetyl cholinesterase activity which reflects its influence on the cholinergic system.
IntroductionEnvironmental toxicants are chemicals found in the environment which are harmful to humans and wildlife. Many chemicals used by humans are dispersed in the ecosystem. Mechanisms by which environmental toxicants impart toxicity include formation of free radical and alteration of cellular components [1]. Polychlorinated biphenyls (PCBs) are organic pollutants and are very stable in nature. Since the 1970s, when restrictions were imposed on the production of PCBs, concentrations in environment have shown a gradual decrease. But residues of PCBs still persist in air, soil, water, and sediment and can be detected in biologic tissues in most residents of industrialized countries [2,3
4]. Animal experiments have evidences of the acute effects of PCBs on the liver, kidney, and central nervous system upon oral exposure [5]. The toxicity of PCBs is fairly well described, and embraces changes in neurotransmitter levels and its reuptake, calcium homeostasis, intracellular signaling pathway activation, and for some congeners, it is found to cause cancer [6,7]. The neurotoxicity of PCBs is known to arise due to its ability to induce oxidative stress in the brain and also induce formation of reactive oxygen species (ROS) as PCBs are reduced by cytochrome P450 in to products such as semiquinone and hydroquinone [8]. The neurological impact of PCBs have been comprehensively studied in various animal models of PCB exposure, either as single congeners or mixtures, during different periods of the animal's lifespan [9]. Many of these studies were conducted only on young animals or animals exposed prenatally to PCBs [10,11]. However, a study conducted on adult rats found that spatial learning behavior was altered due to PCB exposure, thereby indicating that PCBs can alter the nervous system in adult animals as well [12,13]. Coenzyme Q10 (CoQ10) is a ubiquinone, which is a fat-soluble vitamin-like substance found in bodies every cell and functions as a coenzyme for a number of key enzymatic steps in the generation of energy inside the cell. CoQ10 has a wide variety of functions and applications in the body. It is the coenzyme for mitochondrial enzymes (complexes I, II and III) as well as enzymes in other parts of the cell. Mitochondrial enzymes of the oxidative phosphorylation pathway are crucial for the production of the high-energy phosphate, adenosine triphosphate (ATP), which is the main currency for cellular function [14,15]. CoQ10 per se or in combination with other drug may be valuable in the cure of numerous health problems, mainly cardiac diseases, breast cancer, diabetes mellitus, immune deficiency, muscular dystrophy, and periodontal disease [16]. Decreased levels of CoQ10 has been noted in several diseases both in animals and humans. Especially in diseases linked to oxidative stress like Alzheimer's disease, diabetes, prion disease and carcinogenesis, CoQ10 biosynthesis is increased [17]. Both genetic and environmental mediators contribute in triggering the Parkinson's disease causing energy pathway impairment and oxidative stress. CoQ10 acting as a mitochondrial nutrient and plays an important role in mitigating both these processes [18]. Also there are strong evidences for the absorption and distribution of CoQ10 to neural tissues as well as preliminary evidences for its role as a neuroprotective in animal models [19].
The important findings from the animal literature as well as the epidemiological studies in humans suggest that the minimum exposure of PCBs is sensitive enough to cause behavioral deficit [9,20]. In addition, results from studies carried out in rodents and monkeys shed light on neurotoxic potentials of PCBs [21,22]. Hence, in the light of existing knowledge, the present study was planned to study the impact of CoQ10 on the oxidative stress induced by oral 28 days exposure of aroclor 1254, a PCB mixture in the brains of Swiss albino mice. For this we estimated the activities of the enzymes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), acetyl cholinesterase (AChE) and levels of lipid peroxidation (LPO) and reduced Glutathione (GSH) in the brains of mice to shed light on the possible mechanism, along with histopathological investigations. L-deprenyl was used as the reference standard as it possesses the property to increase survival of neurons [23].
Materials and MethodsDrugs and ChemicalsAll the chemicals used for biochemical assays were obtained from S.D. Fine Chem Ltd. (Mumbai, India), Thomas Baker Chemicals Pvt. Ltd. (Mumbai, India), Hi-media Laboratories Pvt. Ltd. (Mumbai, India). Aroclor 1254 was obtained from Sigma Aldrich Chemicals (St. Louis, MO, USA), CoQ10 was procured and authenticated from M/s. Sterling Biotech Ltd. (Vadodara, India). The standard used for comparison selegiline HCl (L-deprenyl) was obtained from Cipla Ltd. (Mumbai, India). All chemicals used were of analytical grade.
Animals and TreatmentsTwenty four female Swiss albino mice (20-30 g) were used in the study. Animals were housed in standard polypropylene cages with wire mesh top and maintained at 23±2℃ and relative humidity 60±5% with 12 hours light-dark cycle. Animals were fed with commercially available standard rodent pellet diet (Amrut rat and mice feed manufactured by Navi Maharashtra Chakan Oil Mill Ltd., Mumbai, India). Water was provided to the animals ad libitum. Experimental procedures were approved by the Institutional Animal Ethics Committee of Bombay College of Pharmacy. The aroclor 1254 (PCBs) was dispersed in corn oil, while CoQ10 was dissolved in 25 µL corn oil. Standard drug L-deprenyl was dissolved in 0.9% saline. Aroclor 1254 was given orally and CoQ10 and L-deprenyl were administered intraparitoneally. Mice were randomized into four groups. The group 1 consisted of vehicle (corn oil, oral) control animals, group 2 was of animals exposed to aroclor 1254 (5 mg/kg, oral), group 3 consisted of animals treated with CoQ10 (10 mg/kg, intraperitoneal injection [IP]) daily followed by aroclor 1254 (5 mg/kg, oral) and group 4 was of L-deprenyl (1 mg/kg, IP) daily followed by aroclor 1254 (5 mg/kg, oral). This administration was done for a period of 28 days. The last dose was administered 24 hours before euthanasia.
Preparation of HomogenateAfter 28 days, experimental and control animals were euthanized in CO2 chamber. Brains were immediately taken out and washed thoroughly with ice-cold saline to remove blood. Whole brain was homogenized in 50 mM phosphate buffer pH 7.4 with homogenizer fitted with Teflon plunger. 10% tissue homogenate (w/v) was made. Supernatant was made by centrifuging homogenate at 10,000 rpm for 20 minutes. Homogenate were used for the estimation of LPO and GSH, while supernatant were used for the assay of CAT, SOD, GPx, and AChE activities.
Lipid PeroxidationThe brain tissue homogenate (0.1 mL) was mixed with acetic acid (20%, 1.5 mL), sodium lauryl sulfate (0.2 mL) and thiobarbituric acid (1.5 mL). After incubating at 95℃ for 1 hour the mixture was cooled and 5 mL of n-butanol and pyridine (15:1) were added, shaken vigorously and centrifuged. The absorbance of the homogenate was read at 532 nm against the blank containing all reagents except the homogenate. The inhibition of LPO was determined by the procedure previously described [24]. The values were expressed as nmol of malondialdehyde/mg of protein.
Reduced GlutathioneTo 250 µL of brain tissue homogenate, 0.5 mL of 5% trichloroethanoic acid in 1 mM ethylenediaminetetraacetic acid (EDTA) was added. The sample was centrifuged at 2,000 g for 10 minutes. The supernatant was mixed with 2.5 mL of 0.1 M phosphate buffer (pH 8.0) and 100 µL of (0.001%) 5, 5'-dithiobis-2-nitrobenzoic acid (DTNB). Absorbance was read at 412 nm. Values were calculated from a standard graph of GSH treated with same reagent [25]. The amount of reduced glutathione was expressed as µg of GSH/mg protein.
Catalase AactivityThe procedure followed was as discussed previously [26]. The reaction mixture consisted of 2 mL phosphate buffer (pH 7.0), 0.95 mL of hydrogen peroxide (0.019 M) and 0.05 mL of supernatant in a final volume of 3 mL. Absorbance were recorded at 240 nm every 10 seconds for 1 minute. The results were expressed as units of CAT activity/mg protein.
Superoxide Dismutase ActivityThe reaction mixture consisted of 0.05 mL of supernatant, 2 mL of carbonate buffer and 0.5 mL of EDTA solution. The reaction was initiated by the addition of 0.5 mL of epinephrine and the autoxidation of adrenaline to adrenochrome at pH 10.2 was measured by following the change in optical density at 480 nm. Change in optical density every minute was noted at 480 nm against reagent blank. The results were expressed as units of SOD activity/mg protein [27].
Glutathione Peroxidase ActivityThe reaction mixture contained 0.5 mL of phosphate buffer (20 mM, pH 7.0), 0.1 mL of glutathione reductase (0.24 U), and 0.1 mL of 10 mM GSH and 0.05 mL of supernatant. Sodium azide (1 mM) was added to the reaction mixture in order to inhibit remnant CAT activity. Thereafter 0.1 mL nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) solution (0.118 mM) was added and the hydroperoxides independent consumption of NADPH was monitored for about 3 minutes. Addition of 0.1 mL hydrogen peroxide (12.5 mM) started the overall reaction and the decrease in absorption at 240 nm was monitored for 5 minutes. The results were expressed as units of GPx activity/mg protein [28].
Acetylcholinesterase ActivityTo a cuvette containing 2.6 mL of phosphate buffer (pH 7.5, 0.1 M), 0.4 mL supernatant was added. 100 µL of DTNB reagent was added. The absorbance was measured at 412 nm, when it stopped increasing, absorbance was set to zero. 20 µL of acetylthiocholine was added. Changes in absorbance were recorded for 3 minutes and the change in absorbance per min was calculated. The results were expressed as AChE nmol per minute per g tissue of brain [29].
Histopathological EvaluationsThe cerebral cortex was fixed in 10% formalin. The specimens were then processed by standard procedure. The blocks were then sectioned according to hematoxylin and eosin method [30]. Histological sections of five micrometer were taken. The sections were examined under the light microscope and photographed at 100× using Motic camera (BA310; Hong Kong).
ResultsLipid PeroxidationThe malondialdehyde levels, an indicator of LPO were increased in the brain of mice treated with aroclor 1254 (1.85 nmol/mg protein as compared to normal group 0.68 nmol/mg protein). This increase was abated by 10 mg/kg IP dose of CoQ10 (0.55 nmol/mg protein as compared to that of L-deprenyl 0.64 nmol/mg protein) (Table 1).
Reduced GlutathioneReduced glutathione, a thiol plays a vital role in protecting the cells during endogenous and exogenous free radicals and oxidants mediated insults. The brain GSH levels in mice on 28 days exposure to aroclor 1254 were decreased to 0.25 µg/mg protein from control levels of 0.76 mg/mg protein. Intraperitoneal administration of CoQ10 (10 mg/kg) increased the levels of GSH to 0.42 µg/mg protein. The increase in GSH content by CoQ10 was comparable to the increase obtained by L-deprenyl (0.52 µg/mg protein) (Table 1).
Superoxide Dismutase ActivitySOD is a measure of the oxidative stress produced in the organ as it dismutase O2 anions. Decrease in its activity directly corresponds to the increase in oxidative stress. The significant reduction of 4.43 units/mg protein seen in the aroclor 1254 exposed group as contrast to the control group (8.74 units/mg protein) CoQ10 (10 mg/kg) intraperitoneal administration increased the levels of SOD (5.90 units/mg protein) as compared to that of L-deprenyl (6.73 units/mg protein) (Table 1).
Catalase ActivityThe ability of catalase to induce reduction in levels of hydrogen peroxidase was evaluated. Hydrogen peroxidase gives rise to free radicals which are congeners of oxidative stress in our body. The exposure of aroclor 1254 for 28 days resulted in a significant decrease in catalase activity to 0.98 units/mg protein. Supplementation with intraperitoneal CoQ10 increases this activity to 2.21 units/mg protein, which is comparable to L-deprenyl treated group (3.23 units/mg protein) (Table 1).
Glutathione Peroxidase ActivityGPx is mainly responsible for alleviating the effects of free radicals in brain. The decrease in its activity in aroclor 1254 exposed group 3.82 units/mg protein as compared to vehicle control group (19.23 units/mg protein) bears testimony to the oxidative stress inducing effects of aroclor 1254. Co administration of CoQ10 moderately prevented the dramatic decrease in activity (6.98 units/mg protein). The L-deprenyl group also showed an improvement in the enzyme activity (10.70 units/mg protein) (Table 1).
Acetylcholinesterase ActivityAcetylcholinesterase is the main enzyme in brain causing degradation of acetylcholine, which is involved in many motor coordination skills and also memory. The AChE activity of vehicle control group was (230 nmol/min/tissue). The decrease in activity of acetyl cholinesterase (136 nmol/min/tissue) on exposure to aroclor 1254 for 28 days was alleviated on treatment with intraperitoneal CoQ10 treatment (194 nmol/min/tissue). This value bears same level of significance as that obtained with the administration of standard L-deprenyl (219 nmol/min/g tissue) (Table 1).
Histopathological EvaluationHistopathological evaluation of sections of cerebral cortex of mice brains exposes degeneration, congestion and infiltration in aroclor 1254 exposed group (Figure 1A), as compared to vehicle control group with normal morphology (Figure 1B). Significant prevention of aroclor exposed structural derangements in the cerebral cortex was seen in CoQ10 treated group (Figure 1C) which was comparable to the standard L-deprenyl treated group (Figure 1D).
DiscussionIn relation to other organs of the body, the brain is predominantly susceptible to oxidative damage as it undergoes aerobic metabolism and use 20% of the oxygen, generating large amount of ATP molecules [31,32]. It also contains relatively high amount of polyunsaturated fatty acids which are easily oxidizable. Moreover owing to the lipophilic nature of the environmental toxicants, they gain easy access to the brain and hence brain becomes more vulnerable to their toxicity. PCBs form reactive metabolites PCB-epoxides and PCB-quinones. These metabolites are capable of binding to the DNA and RNA which are implicated in the neurodegenerative diseases. PCB induced detrimental effects are associated with the generation of free radicals [33]. Previous evidences suggest that dopaminergic neurons are particularly vulnerable to oxidative stress due to production of ROS due to inherent auto oxidation of dopamine to form H2O2 superoxide anion and dopamine quinines [34,35]. These oxygen radicals can react with cellular nucleophiles, such as GSH. Oral administrations of PCB to rats and primates have been shown to decrease dopamine levels in the mammalian brains in vivo [21]. While a study unraveled that, 52 weeks of exposure to several aroclor mixtures at a high dose in the diet was unsuccessful in producing any functional or morphologic changes indicative of PCB-induced neurotoxicity [36]. On the contrary, the current study shows that sub chronic exposure of aroclor 1254 in mice produced significant neurotoxicity. Many previous studies investigated and reported the role of CoQ10 as neuronal protectant against ROS induced damage and apoptotic cell death. CoQ10 possibly acted through mitochondrial membrane stabilization, when neuronal cells are subjected to oxidative stress [18]. In the present study we investigated the role of CoQ10 in alleviating the oxidative stress induced by oral exposure to aroclor 1254 for 28 days in the brains of Swiss albino mice. The oxidative stress and the free radical generated by the biphenyls are due to the formation of the LPO products leading to the aberrations of neuronal membrane structure. The decrease in activities of radical scavenging enzymes like SOD, CAT, and GPx and levels of GSH resulted in increased oxidative stress in brain. These mechanisms contribute to the PCBs induced neurotoxicity. This investigation revealed a significant decrease in activities of SOD, CAT, GPx and the GSH levels, while a complementary increase in lipid peroxidation was observed on sub acute exposure to aroclor 1254. CoQ10 (5 mg/kg, IP, for 28 days) treatment significantly reversed the detrimental oxidative changes in brain induced by PCBs exposure.
Acetylcholine is known to play an important role in motor coordination and its altered levels are implicated in many neurodegenerative disorders. AChE influences cholinergic transmission and it is known that its activity is inhibited by free radical formation [37]. Motor coordination and cognition is primarily mediated through cholinergic transmission. It is reported that PCB exposure impacts cholinergic system in experimental animals [38]. Our study revealed that intraperitoneal administration of CoQ10 (5 mg/kg, 28 days) treatment significantly abated the changes in anticholinesterase activity induced by aroclor 1254 exposure. Earlier reports have indicated that L-deprenyl indirectly inhibits AChE, but the underlying mechanism is unclear [39]. In the present study L-deprenyl restored the activity of AChE to normal levels. Further, the histopathological evidences of the cerebral cortex of mice brains corroborate the protection offered by CoQ10 in countering the neurotoxicity of PCBs.
We conclude that aroclor 1254 induced oxidative stress mediated neurotoxicity. CoQ10 restored the levels of GSH and the activities of SOD, CAT, GPx, AChE that was adversely wavered by the PCB mixture aroclor 1254 and prevented LPO in the brain region. The treatment outcome obtained is comparable with that of L-deprenyl. These results depict the neuroprotective action of CoQ10 against aroclor 1254 induced brain toxicity.
Conflict of interestThe authors have no conflicts of interest with material presented in this paper.
References1. Wright DA, Welbourn P. Environmental toxicology. Cambridge: Cambridge University Press; 2002. p. 70.
2. Letcher RJ, Lemmen JG, van der Burg B, Brouwer A, Bergman A, Giesy JP, et al. In vitro antiestrogenic effects of aryl methyl sulfone metabolites of polychlorinated biphenyls and 2,2-bis (4-chlorophenyl)-1,1-dichloroethene on 17 beta-estradiol-induced gene expression in several bioassay systems. Toxicol Sci 2002;69(2):362-372. 12377985.
3. McKinney MA, De Guise S, Martineau D, Béland P, Arukwe A, Letcher RJ. Biotransformation of polybrominated diphenyl ethers and polychlorinated biphenyls in beluga whale (Delphinapterus leucas) and rat mammalian model using an in vitro hepatic microsomal assay. Aquat Toxicol 2006;77(1):87-97. 16325935.
4. Verreault J, Muir DC, Norstrom RJ, Stirling I, Fisk AT, Gabrielsen GW, et al. Chlorinated hydrocarbon contaminants and metabolites in polar bears (Ursus maritimus) from Alaska, Canada, East Greenland, and Svalbard: 1996-2002. Sci Total Environ 2005;351-352: 369-390. 16115663.
5. US Agency for Toxic Substances and Disease Registry. Research Triangle Institute. Toxicological profile for polychlorinated biphenyls. Atlanta, GA: US Agency for Toxic Substances and Disease Registry; 1997. p. 6-23.
6. Fonnum F, Lock EA. The contributions of excitotoxicity, glutathione depletion and DNA repair in chemically induced injury to neurones: exemplified with toxic effects on cerebellar granule cells. J Neurochem 2004;88(3):513-531. 14720201.
7. Tilson HA, Kodavanti PR. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology 1998;19(4-5):517-525. 9745906.
8. Monks TJ, Lau SS. Toxicology of quinone-thioethers. Crit Rev Toxicol 1992;22(5-6):243-270. 1489507.
9. Faroon O, Jones D, de Rosa C. Effects of polychlorinated biphenyls on the nervous system. Toxicol Ind Health 2000;16(7-8):305-333. 11693948.
10. Campagna R, Brunelli L, Airoldi L, Fanelli R, Hakansson H, Heimeier RA, et al. Cerebellum proteomics addressing the cognitive deficit of rats perinatally exposed to the food-relevant polychlorinated biphenyl 138. Toxicol Sci 2011;123(1):170-179. 21673325.
11. Boucher O, Muckle G, Bastien CH. Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ Health Perspect 2009;117(1):7-16. 19165381.
12. Newland MC. Neurobehavioral toxicity of methylmercury and PCBs: effects-profiles and sensitive populations. Environ Toxicol Pharmacol 2002;12(2):119-128. 21782631.
13. Holene E, Nafstad I, Skaare JU, Sagvolden T. Behavioural hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav Brain Res 1998;94(1):213-224. 9708851.
14. Beal MF. Therapeutic effects of coenzyme Q10 in neurodegenerative diseases. Methods Enzymol 2004;382: 473-487. 15047118.
15. Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion 2007;(7 Suppl):S41-S50. 17482888.
16. Crane FL. Discovery of ubiquinone (coenzyme Q) and an overview of function. Mitochondrion 2007;(7 Suppl):S2-S7. 17446142.
17. Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta 2004;1660(1-2):171-199. 14757233.
18. Mancuso M, Orsucci D, Calsolaro V, Choub A, Siciliano G. Coenzyme Q10 and neurological diseases. Pharmaceuticals 2009;2(3):134-149.
19. Kaczor T. Coenzyme Q10 in Parkinson's disease: ready for first line use? Nat Med J 2010;2(11):3-7.
20. Carpenter DO, Hussain RJ, Berger DF, Lombardo JP, Park HY. Electrophysiologic and behavioral effects of perinatal and acute exposure of rats to lead and polychlorinated biphenyls. Environ Health Perspect 2002;110(Suppl 3):377-386. 12060832.
21. Seegal RF, Bush B, Brosch KO. Sub-chronic exposure of the adult rat to Aroclor 1254 yields regionally-specific changes in central dopaminergic function. Neurotoxicology 1991;12(1):55-65. 2014069.
22. Muthuvel R, Venkataraman P, Krishnamoorthy G, Gunadharini DN, Kanagaraj P, Jone Stanley A, et al. Antioxidant effect of ascorbic acid on PCB (Aroclor 1254) induced oxidative stress in hypothalamus of albino rats. Clin Chim Acta 2006;365(1-2):297-303. 16248992.
23. Todd KG, Butterworth RF. Increased neuronal cell survival after L-deprenyl treatment in experimental thiamine deficiency. J Neurosci Res 1998;52(2):240-246. 9579414.
24. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-358. 36810.
25. Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 1979;582(1):67-78. 760819.
26. Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 1952;195(1):133-140. 14938361.
27. Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem 1978;90(1):81-89. 727489.
28. Carrillo MC, Kanai S, Nokubo M, Kitani K. (-) deprenyl induces activities of both superoxide dismutase and catalase but not of glutathione peroxidase in the striatum of young male rats. Life Sci 1991;48(6):517-521. 1899460.
29. Ellman GL, Courtney KD, Andres V Jr, Feather-Stome RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7: 88-95. 13726518.
30. Culling CA. Handbook of histopathological and histochemical techniques: including museum techniques. 3rd ed. London: Butterworth; 1974. p. 361.
31. Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res 2007;35(22):7497-7504. 17947327.
32. Cannon JR, Greenamyre JT. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci 2011;124(2):225-250. 21914720.
33. Tharappel JC, Lee EY, Robertson LW, Spear BT, Glauert HP. Regulation of cell proliferation, apoptosis, and transcription factor activities during the promotion of liver carcinogenesis by polychlorinated biphenyls. Toxicol Appl Pharmacol 2002;179(3):172-184. 11906247.
34. Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. J Neurochem 1999;73(3):1127-1137. 10461904.
35. Nikolova G. Oxidative stress and Parkinson disease. Trakia J Sci 2012;10(1):92-100.
36. Freeman GB, Lordo RA, Singer AW, Peters AC, Neal BH, McConnell EE, et al. An assessment of neurotoxicity of aroclors 1016, 1242, 1254, and 1260 administered in diet to Sprague-Dawley rats for one year. Toxicol Sci 2000;53(2):377-391. 10696786.
37. Tsakiris S, Angelogianni P, Schulpis KH, Stavridis JC. Protective effect of L-phenylalanine on rat brain acetylcholinesterase inhibition induced by free radicals. Clin Biochem 2000;33(2):103-106. 10751587.
Figure 1.Photomicrographs of histopathological slides (100×) of mice cerebral cortex. (A) Aroclor 1254 exposed group shows congestion (c), degeneration (d), and infiltration. (B) Vehicle control group treated confirms normal architecture. Ca, capillary; N, nerve cell. (C) Coenzyme Q10 treated group significantly reverses the lesions due to aroclor 1254. (D) Very mild congestion found in standard L-deprenyl exposed group. 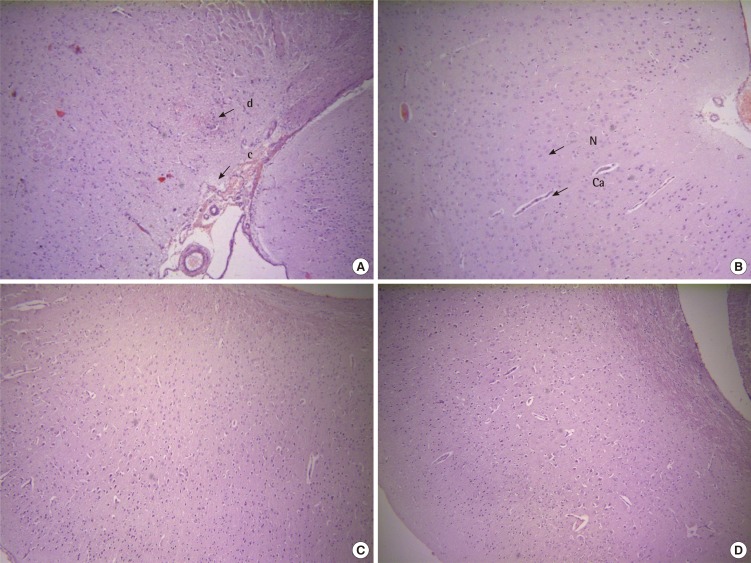
Table 1.Effect of CoQ10 on antioxidant markers in aroclor 1254 induced oxidative stress in mice brain
Statistical analysis was carried out using one way ANOVA followed by Bonferroni’s multiple comparison post hoc test. |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||