Introduction
Human activity and industrialization have led to the development and use of a variety of toxic chemicals. Among the chemical substances emitted into the environment, those substances that affect the endocrine system have been the focus of much research [1-3].
While endocrine disrupting chemicals (EDCs) do exist in the natural world, many have been artificially created. They are known to cause reproductive problems and population decline when absorbed inside living organisms through their disruption of the endocrine system.
EDCs, such as nonylphenol (NP), bis-phenol A (BPA), and phthalates, are used in the manufacturing of plastic, phenol resin, epoxy resin, and polycarbonate resin. The consumption of these substances increases yearly, along with their emission into the environment. Estrogen produced within the body breaks down inside the body after a certain period of time, whereas artificially created estrogenic chemicals do not break down and remain active. Even exposure to small quantities can have an effect.
Inside the body, EDCs typically activate estrogen responsive genes such as vitellogenin (VTG) by binding with estrogen receptors [4,5].
The VTG of oviparous animals is synthesized in the liver when induced by estrogen.
After moving to the ovary through the blood vessels, it is used in the formation of the oocyte through the process of vitellogenesis.
While VTG is not normally detected in male or immature female animals, expression can be induced by typical EDCs such as NP or BPA [6,7]. Accordingly, the increase of VTG in males or immature females can be evidence of exposure to EDCs, and it is widely recognized that VTG can be used as a biomarker for EDCs.
It has been reported that the expression of high levels of VTG in male fish caught in rivers or creeks can occur due to the presence of chemical substances such as estrogen in the water [8]. VTG can be measured via methods such as radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), immunodiffusion, and alkali-labile phosphate [9].
Among these, RIA and ELISA are the methods primarily used. RIA, which labels VTG with radioactive isotopes using methods established in 1979 [10], is difficult, and not very safe nor economic. ELISA does not deal with radioactive material and is safe.
In order to evaluate the endocrine disruption effect of new chemical substances using VTG, many researchers are in the ongoing process of development of an ELISA system using small fish species such as the pale chub (Zacco platypus), fathead minnow (Pimephales promelas), zebrafish (Danio rerio), and Japanese medaka (Oryzias latipes).
The pale chub (Zacco platypus) is a highly migratory species mainly found in the Asian regions of China, Japan, and Korea [11], and primarily eats insects, algae, sediment, and microcrustaceans [12].
Due to its food chain and habitat, the pale chub can be exposed directly and indirectly to a variety of environmental pollutants. The river environment can be monitored by the pale chub's life cycle, making it an excellent species for a model of toxicity effects. Therefore, the development of an ELISA system using the pale chub is needed in order to measure the change in VTG in fish species exposed to EDCs in Korean rivers.
Existing pale chub ELISA systems [13] require the sacrifice of a great number of fish to produce the needed antibodies from blood. However, with the introduction of the 3R (reduction, refinement, replacement) concept concerning animal welfare, policies have been implemented such as the development of new experimental methods that substitute for test animals, reduce the use of test animals, or reduce the sacrifice of animals through the improvement of experimental methods [14].
In this study, instead of antibodies produced from blood, we attempt to quantify VTG while reducing the number of fish used in experiments through the fabrication of antibodies from ova. VTG, a precursor of yolk protein, is converted to lipovitellin and phosvitin inside the egg [15].
After this, a chemical reaction inside the egg causes the vitellin (Vn) to separate, where it is used in the formation of the egg. Therefore, Vn, being separated from VTG, has the same antigenicity and can be used instead of VTG. In addition, Vn is stored in the egg where a large amount is concentrated. This means that we can procure a large amount from a small number of fish compared with separating it from blood.
In this study, instead of collecting blood from many fish, by extracting the ova from female fish and producing monoclonal antibodies (mAb) and polyclonal antibodies (pAb), we minimized the number of specimens needed for the experiment. By applying these antibodies in a sandwich ELISA system, we seek to develop a new ELISA method for quantifying pale chub VTG.
Materials and Methods
Pale Chub Sampling
The pale chubs used in this study were collected using a net in the upstream part of the Gap River in the Korean city of Daejeon, and specimens over 10 cm long were selected to be used.
To separate the ovaries and blood from the pale chubs, 150 mg/L of tricaine methanesulfonate (MS-222; Sigma-Aldrich, St. Louis, MO, USA) was used to anesthetize the specimens. The ova were separated from the ovaries, washed in a physiological saline solution, and stored in a freezer at -80℃ until the yolk protein was separated. The blood was drawn using a syringe (23 G) pre-treated with heparin, and the serum was centrifuged for 30 minutes at 3,000×g and stored in a freezer at -80℃ until the experiment was conducted.
Separation of Vitellin and Vitellogenin
Following the methods of [16,17], Vn was extracted from the ovary of a mature female pale chub. The ovum was separated from the ovary, and 5 mL of 20 mM Tris-HCl buffer (pH 8.0) combined with 0.5 M NaCl was added. Once pulverized with a homogenizer (Cole-Parmer, Vernon Hills, IL, USA), it was centrifuged at (10,000×g, 1 hour, 4℃) and the supernatant was collected.
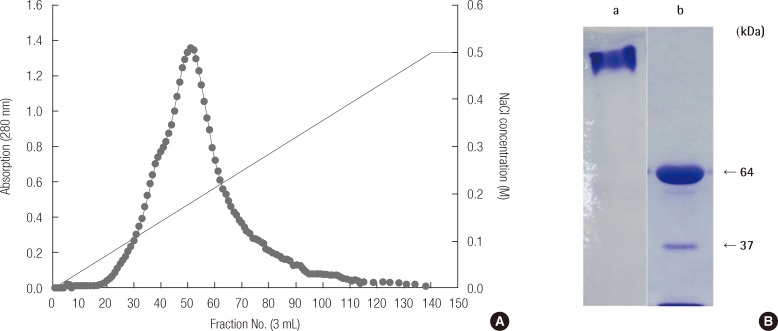
Ammonium sulfate was added to a 0.7 volume of supernatant, and once it had completely melted at 4℃, it was centrifuged at 4℃ for 30 minutes at 10,000×g. The supernatant was then discarded and 0.5 M NaCl was added to a 20 mM Tris-HCl buffer (pH 8.0) containing 0.1% NaN3, 0.01% ethylenediaminetetraacetic acid, 1 mM phenyl methane sulfonyl fluoride in the precipitate. After it was completely dissolved, a DE-52 column (Pharmacia LKB, Uppsala, Sweden) was used to separate the serum protein into several fractions through a linear salt gradient of 0.0-0.05 M NaCl and the absorbance at 280 nm was measured. We determined the protein size of the separated fractions using sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and stored them in a freezer at -80℃ until the assay.
For the VTG induction within the blood, male pale chubs were given intraperitoneal injections 4-5 times at three day intervals of 2 mg of estradiol-17β (E2) per kg of body weight. After treatment, blood was drawn from a caudal fin artery and centrifuged at 4℃ for 30 minutes at 3,000×g. This was stored in a freezer at -80℃ until it was used in the experiment.
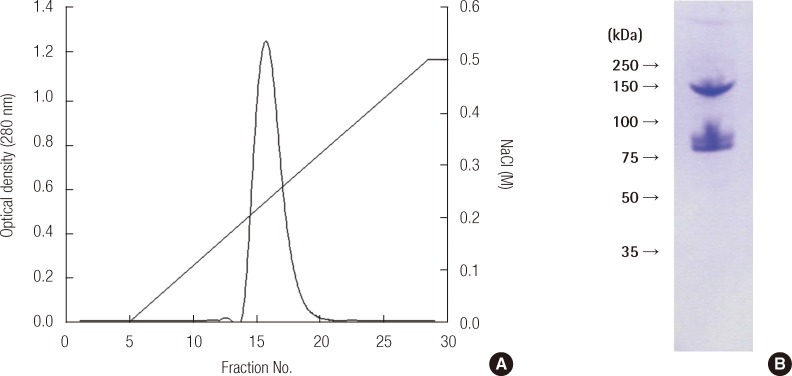
After precipitating protein compounds using ammonium sulfate in the VTG serum, we separated them with a Mono Q HR anion-exchange chromatography column (GE Healthcare, Bucks, UK) using a fast protein liquid chromatography system. The identity of the separated VTG was confirmed with SDS-PAGE.
Antibody Production
The mAb to pale chub Vn, which possesses the same antigenicity as the VTG in a female fish's blood, was produced by modifying the method of Harlow et al. [18]. Separated pale chub Vn was injected into mice. After separating spleen cells that were obtained from immunity induction, they were fused to a myeloma cell line (SP2/0-Ag14) to produce hybridoma cells.
The fused cells were diluted in RPMI-1640 (Gibco, Gran Island, NY, USA), 15% fetal bovine serum (Gibco), and HAT medium (Gibco; 0.1 mm hypoxantine aminopterin, 0.016 mM thymidine), and incubated at 37℃ and 5% CO2, after which the hybridoma cells were separated out.
To confirm the creation of antibodies, we examined whether antibodies had been raised against VTG after 15 days of incubation using a western blot that targeted the supernatant of the well that had formed a colony [19]. The produced mAb was stored in a freezer at -80℃ until its use in the VTG ELISA system.
To produce pAb, separated pale chub Vn was mixed with an equal volume of Freund's complete adjuvant (Sigma-Aldrich) and injected into a rabbit's lymph gland. After inducing immunity, the separated pale chub Vn was mixed with Freund's incomplete adjuvant (Sigma-Aldrich) and injected hypodermically twice in one week increments. Once all the blood was drawn from the carotid artery, it was centrifuged at 3,000×g and the antiserum separated. The separated antiserum was examined using western blotting and stored in a freezer at -80℃ until its use in the VTG ELISA system.
Enzyme-linked Immunosorbent Assay Test Procedure
The produced mAb were diluted in a coating buffer (50 mM sodium carbonate solution, pH 9.6). After flushing a 96-well plate (Immunoplate Maxisorp F96; Nunc, Roskilde, Denmark) with 100 µL per well, the wells were thoroughly sealed and allowed to react at 4℃ for 16 hours. The mAb not coated on the well surface were cleansed 3 times at 5 minute intervals in a washing buffer (0.05% Tween-20 in 0.05 M phosphate buffer saline) and removed.
To block non-specific binding, each well plate was flushed with 200 µL of borate-buffered saline (BBS) (1% bovine serum albumin [BSA] in BBS), sealed well, and allowed to react at 37℃ for 2 hours. Next, it was flushed with 200 µL of a washing buffer, left to stand for 5 minutes, and the buffer was disposed of, three times.
The VTG standard along with the samples were diluted in an antibody dilution buffer (1% BSA in washing buffer). Each well was flushed with 100 µL of diluted VTG standard and thoroughly sealed and allowed to react at 37℃ for 2 hours. The plate was then cleansed with a washing buffer three times.
The pAb were diluted in an antibody dilution buffer, each well of the 96-well plate was flushed with 100 µL and sealed well, and reacted at 37℃ for 2 hours. They were then flushed with 200 µL washing buffer, allowed to stand for 5 minutes, and the buffer was disposed of three times.
Next, goat anti-mouse IgG biotin conjugate (Sigma-Aldrich) was diluted in an antibody dilution buffer. After flushing each well with 100 µL, they were sealed to prevent evaporation and reacted at 37℃ for 1 hour. They were each flushed with 200 µL, left to stand for 5 minutes, and the buffer was disposed of three times.
After diluting ABC reagent (Elite Kit; Vector Laboratories, Burlingame, CA, USA) with a washing buffer and flushing each well with 100 µL of the mixture, the wells' contents were reacted at room temperature for 30 minutes. Then, each well was flushed with 200 µL of washing buffer, left to stand for 5 minutes, and disposed of three times.
A tetramethylbenzidine enzyme substrate (Sigma-Aldrich) was diluted in a citrate-phosphate buffer (pH 5.2), and each well was flushed with 100 µL of the mixture and reacted for 30 minutes in the dark. Thirty minutes later, each well was flushed with 50 µL of 0.5 M H2SO4 to stop the reaction. The optical density was measured using a microplate reader (BioTek, Winooski, VT, USA).
Optimum Conditions and Feasibility Study of Enzyme-linked Immunosorbent Assay System
In order to conduct this study, we established the optimal conditions for the concentrations, specificity, recovery, and intra-assay and inter-assay variations. To determine the optimal concentrations of antibodies, we varied the mAb and pAb dilution concentrations and compared and analyzed 4 concentration ratios: 4,000 vs. 5,000, 4,000 vs. 10,000, 2,000 vs. 5,000, and 2,000 vs. 10,000.
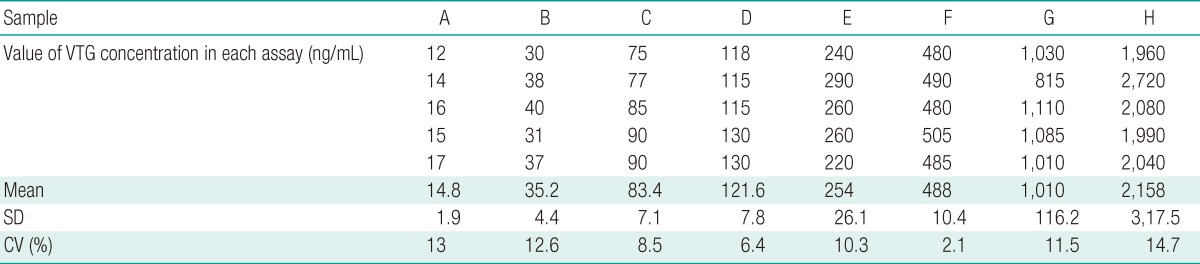
To produce the standard curve, VTG was diluted to concentrations of 15.6, 31.3, 62.5, 125, 250, 500, 1,000, and 2,000 ng/mL. The recovery rate was measured through the injection of VTG extracted from male pale chubs. The measured value was compared with VTG setup concentrations of 0.625, 1.25, and 2.5 µg/mL. The inter-assay coefficients of variation (CV) were determined through 5 standard curves from uniformly diluted samples.
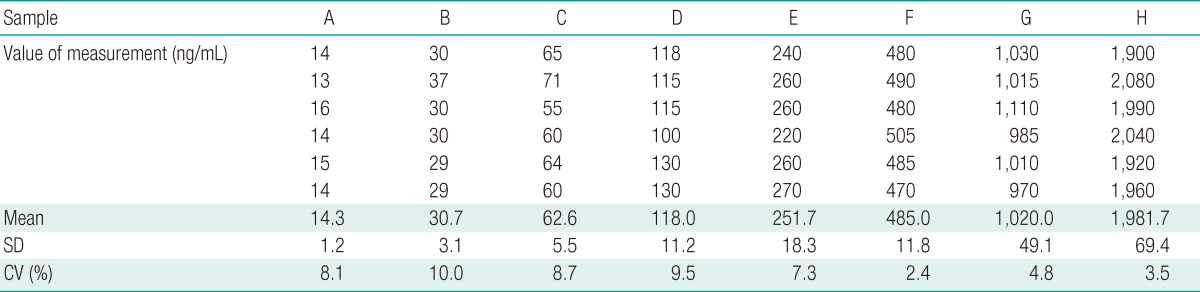
The intra-assay CV were determined based on the value of each concentration through 6 repetitions in each plate.
Results
Vitellin and Vitellogenin Protein Separation and Antibody Production
DE-52 ion exchange chromatography was used to isolate and measure the absorbance of the protein extracted from ova separated from mature female pale chub ovaries. The results of this are shown in Figure 1A. Through an SDS-PAGE of fraction No. 50 of the Vn protein fractions, the results of which are shown in Figure 1B, we were able to determine that the Vn had a protein size of 64 and 37 kDa. Between these, the 64 kDa protein band, considered the primary subunit of Vn, was cleaved and antibodies were produced using mice and rabbits.
VTG from the blood of male pale chubs that had been treated with E2 was isolated using anion exchange chromatography with a Mono Q column and gel-filtration chromatography with an HR 10/30 column. The results are shown in Figure 2A, the main peak was in fraction No. 16. The results from an SDS-PAGE analysis of this showed proteins having a molecular weight of 146 kDa and 77 kDa, similar to those of Figure 2B.
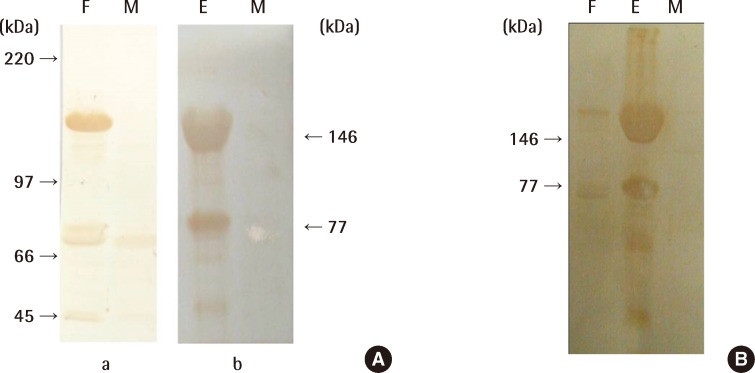
In order to confirm that the produced antibodies were specific to VTG, western blotting was conducted on serum from females, males, and males that had been treated with E2. The results of this are shown in Figure 3.
Figure 3A shows the results for the mAb produced from Vn, confirming that they react specifically with proteins of 146 kDa and 77 kDa, as in Figure 2B. Therefore, we were able to determine that the VTG proteins were composed of two subunits and had the equivalent antigen-determinant sites. In addition, the mAb produced in this study had no immunoreaction with protein from the serum of males that had not been treated with E2, whereas female serum and male serum treated with E2 displayed an immunoreaction.
Accordingly, we were able to confirm that the mAb produced in this study was an antibody to VTG. The immunoreaction results of pAb obtained by injecting a rabbit with Vn separated from pale chubs confirmed in Figure 3B that, as mAb reacts with VTG, it binds specifically to the VTG protein. While it showed an equivalent immunoreaction in mature females and males treated with E2, we saw no immunoreaction with male serum protein. Therefore, we proved that instead of producing antibodies by extracting VTG from pale chub blood, we can detect pale chub VTG by using antibodies produced by separating Vn from ova taken from female pale chub ovaries.
Feasibility Study of Enzyme-linked Immunosorbent Assay System for Vitellogenin Quantity
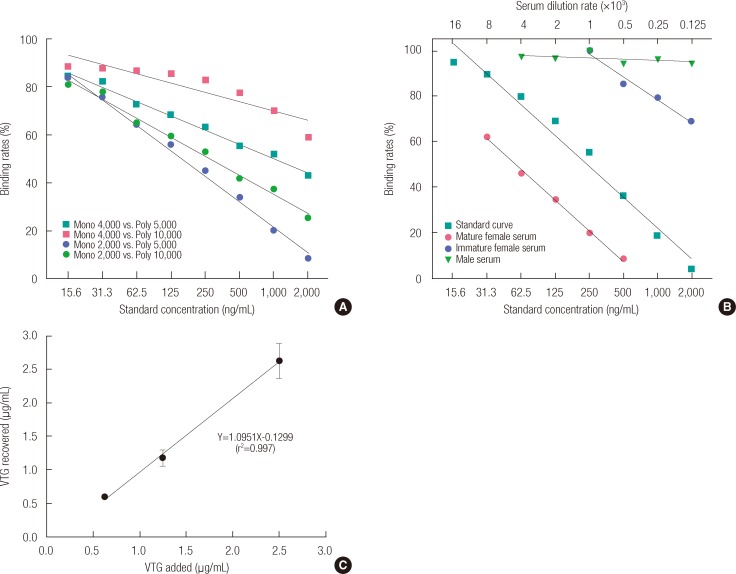
The antibodies used in this study were produced from ova extracted from mature female ovaries. We experimented with a variety of concentrations, looking for the appropriate dilution ratio of mAb and pAb in order to determine the optimal concentration of antibodies. It was found that the difference from the concentration of the VTG standard could best be distinguished when mAb was at a dilution ratio of 1 vs. 2,000 and pAb at 1 vs. 5,000 (Figure 4A).
In order to determine the specificity and reactivity of the antibodies produced from Vn, the serum of male, mature female, and immature female pale chubs was diluted to a variety of concentrations. As shown in Figure 4B, the binding rate that was found by applying our novel VTG ELISA system was compared with the Vn standard curve.
Figure 4B shows that the binding rate hardly changed with the change in concentration of male serum. However, the results of the experiment diluting the serum of mature females show variation in the purified VTG standard curve and binding rate, but the pattern is similar.
These results show that the Vn antibodies used in the ELISA system for this VTG quantity have a high specificity with VTG from serum.
The intra-assay CV ranged from 2.4 to 10.0% (n=6) (Table 1). The inter-assay CV ranged from 2.1 to 14.7% (n=5) (Table 2). In order to determine the recovery rate for this new VTG ELISA system, we added VTG concentrations of 0.625, 1.25, and 2.5 µg/mL, which produced 0.59±0.02, 1.18±0.12, and 2.63±0.26 µg/mL, respectively. Such results confirmed a high recovery factor between 94 and 105% (Figure 4C).
Discussion
The objective of this research was to develop a sandwich ELISA system to analyze the effect on VTG, which is related to fish reproduction, of environmental exposure to a variety of chemical substances. In this study, we developed an ELISA system that could quantify VTG using antibodies produced from Vn. The commonly used VTG ELISA system makes use of antibodies to VTG produced from VTG in blood serum.
However, a large number of pale chub specimens are required in order to obtain enough blood for the production of antibodies. Therefore, instead of extracting VTG from serum, we extracted Vn from the ova of mature female ovaries, produced antibodies, and confirmed the specificity of the antibodies' reaction to VTG.
In addition, by using mAb and pAb together, with cross-linking between genetically similar species, we were able to overcome the method's weakness of lowered sensitivity. Though it is difficult to produce mAb, it has the advantage of producing a species-specific reaction, and is thus used for sensitive ELISA systems [20]. In this study, after inducing the specific reaction of the VTG in serum using VTG mAb, we established a sandwich ELISA system with pAb that amplified the reactivity.
VTG is hardly produced in normal males, but in pale chub males treated with E2, levels of VTG notably increase. E2-induced VTG proteins have a size of 146 kDa, showing results similar to the 147 kDa of zebrafish VTG proteins [21].
The results of this study show that the pale chub VTG ELISA system had a sensitivity to concentrations between 15.6 ng/mL and 2,000 ng/mL (Table 2). When compared with VTG ELISA systems for Oncorhynchus mykiss (20 ng/mL), Pleuronectes vetulus (10 ng/mL), Solea vulgaris (12 ng/mL), and Zoarces viviparous (5 ng/mL), the sensitivity was found to be similar [22-24].
To determine the proper dilution ratio of the mAb and pAb we produced, they were diluted to a variety of concentrations and analyzed with the ELISA system. Figure 4A shows a variety of results depending on the dilution ratio of mAb and pAb. The best dilution ratios were evidenced by the levels that most clearly distinguished the differences between the standard and tested concentrations.
Thus it was found that the dilution ratios of 1 vs. 2,000 for mAb and 1 vs. 5,000 for pAb produced the most suitable results for application of the VTG ELISA system. Figure 4B shows the results of an experiment on whether antibodies produced with Vn specifically bind to VTG.
When compared with the VTG standard curve, a concentration-based difference could not be identified in the serum of a male without VTG. However, in the case of mature females, even though there was a difference in the results based on the concentrations, we were able to confirm that the difference between the standard curve and binding rate showed a similar pattern.
It seems that the discrepancies from the VTG standard, which had been purified, in the binding rates for the female serum, was due to contamination of the VTG with other substances in the female serum. Nevertheless, from the results of this study, we confirmed that the antibody produced from Vn specifically binds sufficiently to the VTG in pale chub's blood to be applied to a VTG ELISA system.
The intra-assay CV confirmed in this VTG ELISA system ranged from 2.4 to 10.0% and the inter-assay CV from 2.1 to 14.7%. Generally, when the inter-assay and intra-assay CV values are under 15%, the results can be considered reliable.
Furthermore, the findings of this study are similar to the results of other ELISA systems i.e., Oncorhynchus mykiss with an intra-assay CV of 1.75% and inter-assay CV of 3.86%, Pleuronectes vetulus with an intra-assay CV of 6.8% and inter-assay CV of 9.6%, and Zoarces viviparus with an intra-assay CV of 5% and an inter-assay CV of 16% [21-23].
Accordingly, this study decisively shows that VTG can be quantified using antibodies to Vn produced from ova harvested from ovaries instead of VTG from blood serum and that the VTG ELISA system using this method can be applied to quantifying the VTG of pale chubs inhabiting Korean water systems.