Introduction
More than eight million metric tons per year of carbon black is used worldwide, mainly for tire reinforcing purposes [1]. Carbon black and many other materials such as titanium dioxide, amorphous silica, and talc have been generally treated as nuisances and relatively low toxic dusts; all of these dusts are grouped as poorly soluble particles (PSP) of low cytotoxicity. The American Conference of Governmental Industrial Hygienists recommended that its threshold limit value (TLV) be 3.5 mg/m3 in order to minimize complaints from excess dirtiness [2]. The Ministry of Employment and Labor in Korea adjusted its TLV to 3.5 mg/m3 to match this limit [3]. However, recent studies reported that nano-sized carbon black, as well as being a nuisance, could have high toxicity. After a new concept for nanotoxicity was raised (i.e., the toxicity of particles increases as their size decreases), the toxicities of PSPs (especially in the form of nano-sized particles) have been reevaluated [4,5].
It is widely accepted that PSPs can produce severe long-term health effects such as tumors and cardiovascular disease due to the "particle overload" effect at high concentrations that cause a marked inflammatory response [6-8]. Since initial experiments in nanotoxicology were reported in 1990 [9], many additional toxicity studies on nano-sized carbon black have been performed in different experimental systems including cell culture systems [10-12], animal intratracheal instillation systems [13-16], and animal inhalation systems [17-20]. In addition, an epidemiological investigation reported higher mortality among Korean workers in a tire-manufacturing factory due to cardiovascular disease [21]. Heat and overwork in the workplace were suggested as the main causes, but nano-sized carbon black could be a contributory factor. However, the relationship between nano-sized carbon black and cardiovascular disease is not clear, despite reports that nano-sized carbon black caused or aggravated cardiovascular diseases through producing inflammation [22].
The Occupational Safety & Health Research Institute report [21] also draws attention to the obesity/overweight status, diabetes, and hypertension shared by the victims of cardiovascular disease in the tire-manufacturing factory. Nowadays, obesity has reached an epidemic level in most developed countries because of excess caloric intakes and sedentary lifestyles. The risks of vibration-induced disorders and other issues related with occupational safety and health in the workplace are expected to increase among obese/overweight workers [23]. Obese/overweight individuals are also likely to develop various chronic diseases such as lung disease, hypertension, coronary artery disease, and diabetes due to systemic inflammation caused by being obese/overweight [24]. Recent research also suggested that it could lead to greater damage to obese/overweight workers [25,26].
In this study, it was hypothesized that obese/overweight rats may be more susceptible to the toxicity of nano-sized carbon black than normal weight ones, and nano-sized carbon black would exacerbate pulmonary diseases among obese/overweight rats. For this reason, Sprague-Dawley (SD) rats were made mildly obese (i.e., overweight) by keeping them on a high-fat diet, and were then exposed to nano-sized carbon black for 6 hr/d, 5 d/wk over a 4-week period in a nose-only inhalation chamber, and the relationship between obesity and nano-sized carbon particle exposure assessed.
Materials and Methods
Animals
This study was approved by the Animal Ethics Committee to ensure appropriate animal care was used during the research. Five-week-old male specific pathogen-free SD rats were obtained from Central Lab Animal Inc. (Seoul, Korea). Thirty-two rats were adapted for 1-week and allocated into two groups (normal and overweight rats). The rats were fed a high-fat diet ([HFD], 24.0% protein, 24.0% fat, 41.0% carbohydrate; total energy 4.73 kcal/g, DietsD12451; Research Diets Inc., New Brunswick, NJ, USA) or a normal diet (23.6% protein, 11.9% fat, 64.5% carbohydrate; total energy 4.02 kcal/g, LabDiet 5053; PMI Nutrition International LLC, Saint Louis, MO, USA). Normal and overweight rats were further divided into four groups (low, medium, high concentration groups, and the control group) and exposed to aerosolized nano-sized carbon black for 6 hr/d, 5 d/wk over a 4-week period. The animals were examined daily on weekdays to observe any evidence of exposure-related effects, including respiratory, dermal, behavioral, nasal or genitourinary changes. During the adaptation and carbon black exposure period, rats were housed in polycarbonate cages in a room with controlled temperature (23±2℃), humidity (55±7%) and a 12-hour light/dark cycle. Food and water were available ad libitum.
Generation and Exposure of Nano-sized Carbon Black Aerosols
Printex-90, nano-sized carbon black, was obtained from Evonik Degussa (Nordrhein-Westfalen, Germany). The manufacturer specified a primary particle diameter of 14 nm for Printex 90. Printex 90 (0.4 g, 0.8 g, and 1.5 g) was added to 300 mL distilled water to generate carbon black aerosols (low, medium and high concentrations, respectively), with the suspensions sonicated at 1,179 J in a probe-type ultrasonicator (Vibra-Cell VC-750; Sonics & Materials Inc., Newtown, CT, USA). Particle size and the dispersity were measured with a Zetasizer (Nano Z 590; Malvern Instruments, Malvern, UK). These dispersed carbon black suspensions were then aerosolized through an orifice with a diameter of 0.8 mm, and the concentrations of these aerosols were then adjusted by controlling the volumes of air through venturi nozzles: 4 liters per minute for the low concentration, 8 liters per minute for the medium concentration and 11 liters per minute for the high concentration. To make the ventilation rate 15 times per hour, the total flow of the inhalation chamber was adjusted to 20 liters per minute (NITC System; HCT, Icheon, Korea). The size distribution of carbon black aerosols was verified using a small-sized cascade impactor (MiniMOUDI M135; MSP Corp., Minneapolis, MN, USA), which was subsequently confirmed with transmission electron microscopy, i.e., aerosols of carbon black in the chambers were sampled with filters coated with carbon, and visualized under a transmission electron microscope (H-7100FA; Hitachi, Tokyo, Japan) [27]. During experimental periods, carbon black aerosols in inhalation chambers were sampled on membrane cellulose ester filters. The concentrations of carbon black were measured at 620 nm in a UV/visible spectrophotometer (DU-650; Beckman, Fullerton, CA, USA).
Bronchoalveolar Lavage
After anesthetizing the rats with isoflurane (Ilsung Pharmaceuticals Co. Ltd., Seoul, Korea), the tracheae were cannulated. The left lungs were lavaged five times with 3 mL of calcium- and magnesium-free phosphate buffered saline ([PBS], pH 7.4). Bronchoalveolar lavage (BAL) fluids were centrifuged at 1,500 rpm for 10 minutes (Union 32R; Hanil BioMed Inc., Incheon, Korea). The BAL fluid supernatants were stored at -80℃ for later quantifying albumin, lactate dehydrogenase (LDH) activity and cytokine levels. After counting the total cell number with a Coulter Counter (Hemarvet 850; Drew Science, Miami Lakes, FL, USA), lavaged cells were centrifuged at 1,500 rpm for 10 minutes (Cellspin, Hanil) and stained with Diff-Quick solutions. Differential counts of macrophages, lymphocytes, and polymorphonuclear leukocytes (PMNs) were determined by counting approximately 300 cells under a microscope at 200× magnification. Albumin concentrations, LDH activities, and cytokine levels were measured in the BAL fluid. Albumin concentration and LDH activity were measured with a biochemistry analyzer (TBA 20FR; Toshiba Medical, Tokyo, Japan) and cytokine levels were measured using ELISA kits (BD Biosciences, San Jose, CA, USA).
Lymphocyte Culture
Lymphocytes were separated from the axillary lymph node. The separated cells were suspended in Roswell Park Memorial Institute medium supplemented with 100 unit/mL of penicillin/streptomycin and 10% heat-inactivated fetal bovine serum (FBS). Subsequently, the cells were plated in 24-well culture plates (Falcon 3047; BD Biosciences) at a density of 1×106 cells/well, with 200 ng of lipopolysaccharide (LPS) then added. Cells were incubated (18 hours at 37℃) in a humidified atmosphere of 5% CO2 in air and the plate was centrifuged at 1,000 rpm for 20 minutes (Union 32R, Hanil). The supernatants were stored at -80℃ for later analysis of TNF-α and IL-6.
Lung Burden of Carbon Black
The concentrations of carbon black in lung tissues were measured according to the method of Rudd and Storm with some modifications [28]. Lung tissues were minced and digested in 25% KOH/methanol (w/v) at 60℃ overnight. Then, the mixture was spun at 10,000 rpm for 30 minutes (Micro 17R; Hanil). After the supernatants were removed, pellets were re-suspended in 0.1% Brij®L23 (Sigma, Saint Louis, MO, USA) (v/v) and sonicated for 5 second in a probe-type sonicator (Vibra Cell VC-750; Sonics & Materials Inc). Turbidity was measured at a wavelength of 620 nm in a UV/visible spectrophotometer (DU-650; Beckman). The numbers of carbon black lumps in the lungs were counted with a light microscope at 200× magnification.
Histopathology
Left lungs were fixed in a 10% formalin solution containing neutral buffered PBS and embedded in paraffin. After staining with hematoxylin and eosin, the lungs were examined with light microscopy at 200× magnification. To quantify the histopathologic symptoms, the changes were graded as follows: 0 for no change, 1 for mild (approximately 30%) change, 2 for moderate (approximately 50%) change, and 3 for severe (more than 70%) change.
Statistical Analysis
Data were analyzed by one-way analysis of variance followed by post hoc analysis based on Dunnett's test to verify the differences between the control and the carbon black exposed groups. Student's t-tests were carried out to see the differences between normal weight and overweight rats. These statistical analyses were performed using SigmaPlot version 12.0 (Systat Software Inc., San Jose, CA, USA). Differences were considered significant when the p-value was <0.05.
Results
Characterization and Concentrations of Carbon Black Aerosol in Inhalation Chambers
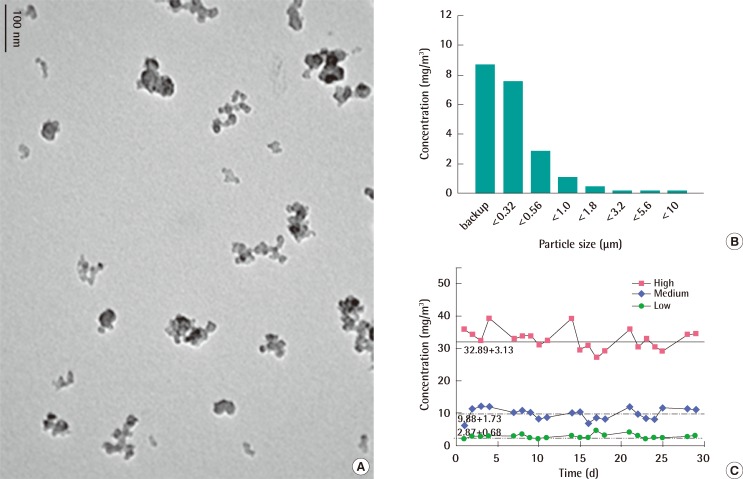
The mean hydrodynamic diameter of nano-sized carbon black particles dispersed in distilled water after sonication was approximately 200 nm with the dispersity being approximately 0.3 and the Z-potential being approximately 17.9 (data not shown). The morphological observation of carbon black particles with transmission electron microscopy (TEM) revealed well-dispersed and little aggregated carbon black particles (Figure 1A). When nano-sized carbon black was aerosolized through an orifice with a diameter of 0.8 mm most carbon black particles were captured in the backup and the 0.32 µm filter (Figure 1B). The mass median aerodynamic diameters of carbon black particles were calculated to be between 0.3 and 0.5 µm with a geometric standard deviation between 2.06 and 3.19 µm (Figure 1B). During the 4-week exposure period, the average concentrations of carbon black aerosols were calculated as 2.87±0.68 mg/m3, 9.88±1.73 mg/m3, and 32.89±3.13 mg/m3 for low, medium, and high concentration groups, respectively (Figure 1C).
Body Weight Changes
The mean body weight of rats fed the HFD increased faster than rats fed the normal diet. At the 9th week, rats fed HFD were overweight or mildly obese. The mean body weight of rats fed the HFD was approximately 15% higher than in those fed the normal diet (Figure 2A). There were no significant differences in the body weight changes between the control and carbon black exposed rats both in normal and overweight rats (Figure 2B).
Inflammatory Responses in BAL
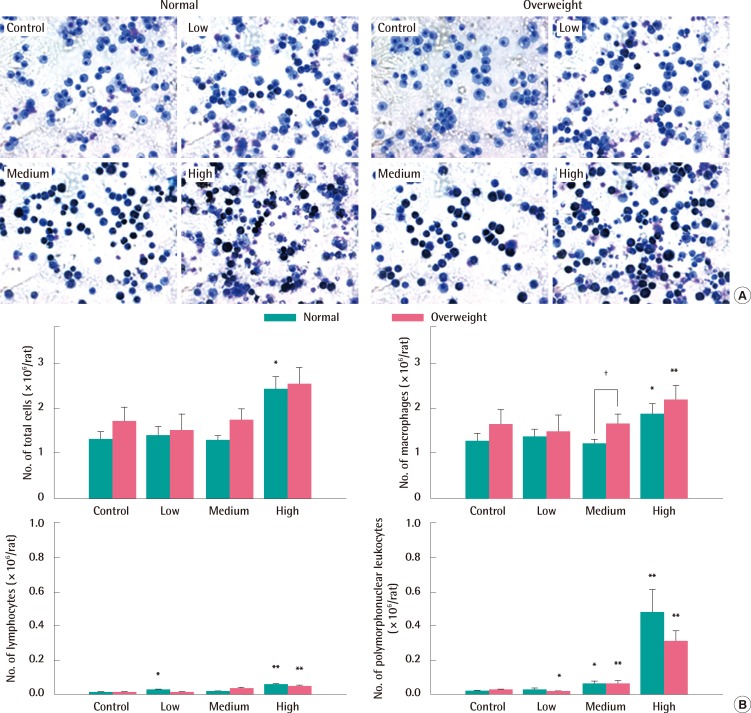
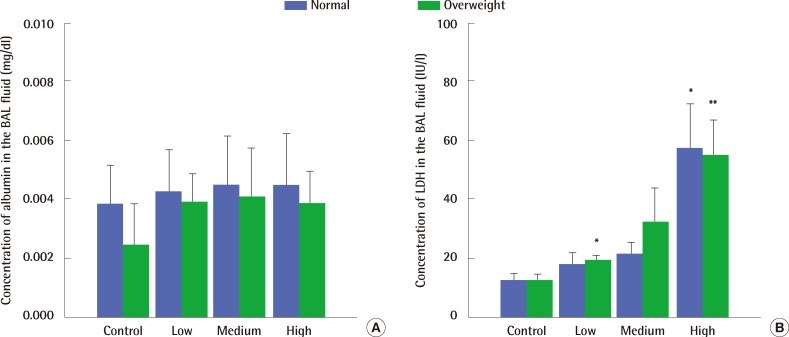
Macrophages taking in carbon black looked black (Figure 3A). Exposure to 9.9 mg/m3 and 32.9 mg/m3 of carbon black aerosols produced significant changes in the number of PMNs compared to control groups of both normal and overweight rats, but there was no significant difference in their number between normal weight and overweight control rats (Figure 3B). Exposure to high concentrations of carbon black increased LDH activity in the BAL fluid (Figure 4A) without any concurrent change in the albumin concentration (Figure 4B). There were no significant differences in both LDH activity and albumin concentrations in the BAL fluid between normal and overweight rats (Figure 4A and 4B).
Lung Burden of Carbon Black and Histopathological Findings
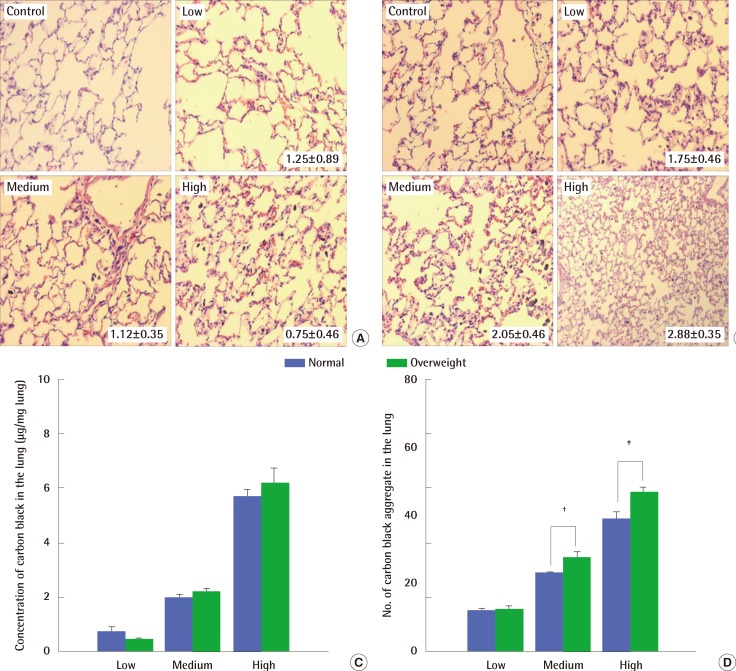
Histological examination of lungs exposed to carbon black showed perivascular infiltration of inflammatory cells, alveolar wall thickening and degeneration, and congestive changes occurring together without fibrosis (Figure 5A and 5B). Mild changes were observed in normal weight rats irrespective of the carbon black concentrations they were exposed to (Figure 5A). In contrast, the histopathological changes were greater in overweight rats at all of the carbon black concentration levels tested (Figure 5B). The deposition of carbon black aggregate in the lungs was a little higher in overweight rats than in normal weight rats (Figure 5C and 5D).
Immunological Responses
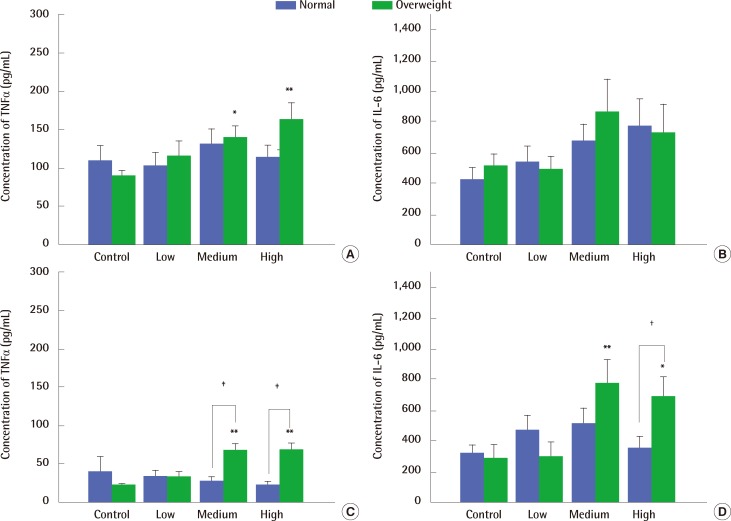
TNF-α levels in the BAL fluid only increased in overweight rats exposed to medium and high concentrations of carbon black (Figure 6A), while IL-6 levels stayed constant regardless of carbon black exposure both in normal- and over-weight rats (Figure 6B). In contrast, TNF-α and IL-6 levels in the supernatant of LPS-stimulated auxiliary lymphocyte cultures taken from overweight rats were significantly increased by exposure to medium and high concentrations of carbon black (Figure 6C and 6D).
Discussion
It was recently reported that obesity could increase workers' sensitivity to chemicals due to a low-grade inflammation of adipose tissues potentially causing chronic activation of the innate immune system by secreting many factors such as leptin, adiponectin, TNF-α, IL-6, etc. [29-31]. In a preliminary test, we confirmed that the high-fat diet-induced obesity rats fed a high-fat diet had significantly elevated serum levels of leptin, total cholesterol, triglyceride and free fatty acid (data not shown).
Generating a well-dispersed and little aggregated/agglomerated aerosol is an important requirement for conducting an inhalation toxicity experiment, with exposure of an enlarged surface area known to cause inflammation, particularly with PSP. The photographs taken with TEM (10,000× magnification) showed that the aerosols were well dispersed compared to previously reported systems: Driscoll et al. [17] generated 0.88 µm aerosols; Elder et al. [18] generated 1.2-2.4 µm aerosols; and Carter et al. [19] generated 1.2-1.6 µm aerosols. As PSPs aggregate rapidly at the workplace and are most likely to be inhaled in this form, the inhalation system used seems to closely simulate the actual exposure conditions of nano-sized carbon black that would normally occur [32].
Pulmonary inflammation is a common response to the inhalation of particles and is closely associated with chronic pathological outcomes [17-19]. For this reason, measuring cell differentiation of BAL cells (i.e., the numbers of total cells, macrophages, PMNs cells and lymphocytes) and cytotoxicity parameters (i.e., LDH activities and albumin concentrations) was undertaken to identify the chronic inflammation caused by nano-sized carbon black. These measures are the preferred parameters in identifying respiratory inflammation induced by particle inhalation, and the Organization for Economic Co-operation and Development guidelines for toxicity tests approve BAL analysis in the subchronic inhalation test [33]. Medium and high concentrations of nano-sized carbon black resulted in significant PMN infiltration compared with the control. As the exposure to high concentrations of nano-sized carbon black has been previously reported to result in cytotoxic damage [17-19], BAL fluid LDH activity was measured as an indicator of cytotoxicity and the albumin concentration was measured as a marker of damage to the pulmonary blood-gas barrier to assess the extent of damage caused by exposure to nano-sized carbon black. Rats exposed to high concentrations of carbon black had significantly higher LDH activities compared to all other treatments, including the control. No significant differences in albumin concentrations were noted between the tested groups. These results were similar to previous studies reporting that mild inflammation occurred at 7 mg/m3, and relatively severe inflammation occurred at 50 mg/m3 after carbon black exposure lasting 6 hr/d, 5 d/wk over a 13-week period [17-19]. Differences in the inflammation and cytotoxic damage between over- and normal-weight rats were not observed under the control conditions.
Clear toxic effects of nano-sized carbon black were found in overweight rats. The lung burden caused by carbon black was higher in overweight rats than in normal weight rats, but a link between high lung burden and severe lung damage in overweight rats has not been verified. The severe damage in overweight rats caused by carbon black resulted in an increased release of proinflammatory cytokines (TNF-α and IL-6) from lymphocyte cultures compared to normal rats, whereas there were no differences in the BAL fluid cytokine levels between treatments.
Overall, inflammation and the damage to lungs in high fat diet-induced overweight rats exposed to nano-sized carbon black were more severe than in normal weight rats. However, the relationship between the activation of proinflammatory cytokines and the lung damage was not established. As such, further more specific studies are needed to investigate the potential relationship between carbon black and cardiovascular disease occurring in tire manufacture workers.